39 orbital diagram of sodium
I think you can safely assume to start off with the molecular orbital diagram of the Nitrite anion (NO₂¯) and then remove an electron from it The energy level diagram is just for understanding how the electrons occupy the orbitals …as said before the bonding electrons occupy first then the antibonding. This video shows how to draw the orbital diagram of Sodium (Na). It also shows how to write the electron configuration of Sodium (Na) and the shorthand noble gas notation as well.
[Original post I made in /r/Astronomy](https://www.reddit.com/r/Astronomy/comments/3fda00/atmospheres_from_stars_to_planets_everything_you/) Hey, /r/space! I recently answered a [question](https://www.reddit.com/r/askscience/comments/3esx20/how_does_pluto_sustain_any_kind_of_atmosphere/) on /r/AskScience about how Pluto can sustain an atmosphere while the Moon does not. This got me thinking that perhaps people would like to know more about atmospheres as an astronomical phenomenon in genera...
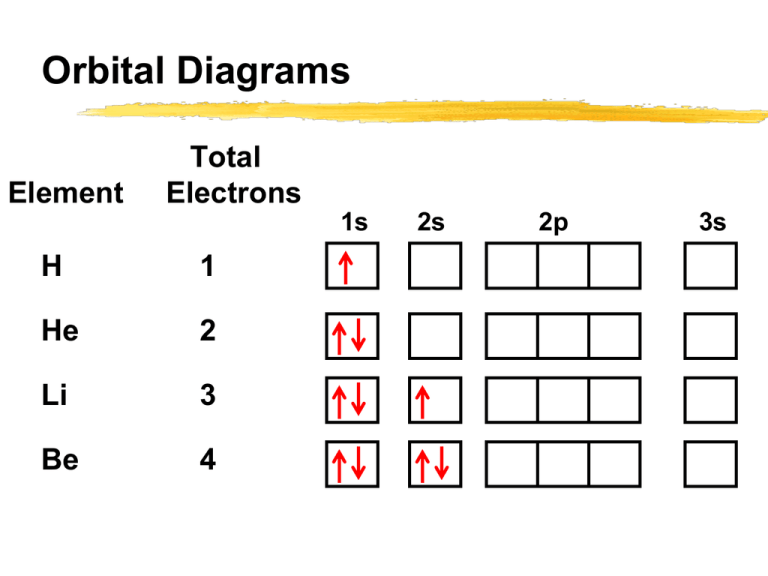
Orbital diagram of sodium
Sodium is a Group 1 element (or IA in older labelling styles). Group 1 elements are often referred to as the "alkali metals". The chemistry of sodium is dominated by Soap is generally a sodium salt of fatty acids. The importance of common salt to animal nutrition has been recognized since prehistoric times. (Recall from Section 5.3B that two electrons in an orbital spin in opposite directions on their axes.) Therefore, if an orbital contains two electrons, its box will contain two arrows, one pointing up and the other down. Using a box diagram, we show the electron configuration of nitrogen as: Notice that the 2p electrons are shown as An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with What orbital do the outmost electrons of sodium fill? The M orbital, there's only 1 electron in it. Why is the size of a sodium ion less that that of a sodium atom?
Orbital diagram of sodium. Nov 23, 2017 · Metals have half filled orbitals. As for example the electron configuration of sodium is 1S 2 2S 2 2P 6 3S 1. Thus sodium has half filled s orbital. Explanation of the electron sea model. Because of low ionization energy, the valence electrons of metals are loosely bonded within the atom. The orbital angular momentum for an atomic electron can be visualized in terms of a vector model where the angular momentum vector is seen as precessing about a direction in space. While the angular momentum vector has the magnitude shown, only a maximum of l units can be measured along a given direction, where l is the orbital quantum number.. Since there is a magnetic moment … Sodium electron configuration is 1s 2 2s 2 2p 6 3s 1.The symbol for sodium is ‘Na’. The period of sodium is 3 and it is a s-block element. This article gives an idea about the electron configuration of sodium(Ne) and orbital diagrams, period and groups, valency and valence electrons of sodium, bond formation, compound formation, application of different principles. Orbital diagram of Sodium. Four physical descriptions - Sodium is so reactive that when it dissolves in water just in a couple of seconds unlike other metals that take time to dissolve.
Hey, /r/astronomy! I recently answered a [question](https://www.reddit.com/r/askscience/comments/3esx20/how_does_pluto_sustain_any_kind_of_atmosphere/) on /r/AskScience about how Pluto can sustain an atmosphere while the Moon does not. This got me thinking that perhaps people would like to know more about atmospheres as an astronomical phenomenon in general. As such I have decided to write a post which will talk about atmospheres of stars and planets in general as well as the atmospheres in our ... Sodium Spectrum The sodium spectrum is dominated by the bright doublet known as the Sodium D-lines at 588.9950 and 589.5924 nanometers. From the energy level diagram it can be seen that these lines are emitted in a transition from the 3p to the 3s levels. The line at 589.0 has twice the intensity of the line at 589.6 nm. is the molecular orbital energy-level diagram for He 2 + . This ion has a total of three valence electrons. Because the first two electrons completely fill the σ 1 s molecular orbital, the Combine the two sodium valence atomic orbitals to produce bonding and antibonding molecular orbitals. Explore more like Molecular Orbital Diagram of Sodium. Valence of Sodium Lewis Structure of Sodium. ©2021 Daily Search Trends Feedback.
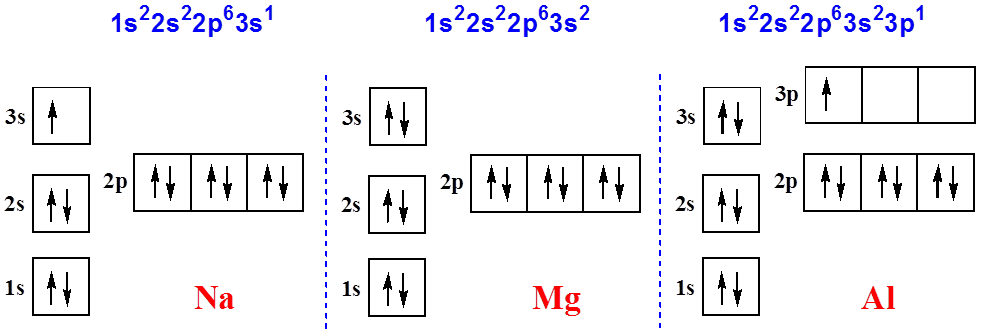
Here you will get the Sodium Electron Configuration (Na) with Orbital Diagram. The symbol of Sodium also provided here. Sodium Electron Configuration: The chemical element sodium has the symbol Na and atomic number 11. This is soft, reactive, silver + whitish metal. Sodium Atomic Emission Spectrum The sodium spectrum is dominated by the bright doublet known as the Sodium D-lines at 588.9950 and 589.5924 nanometers. From the energy level diagram it can be seen that these lines are emitted in a transition from the 3p to the 3s levels. The line at 589.0 has twice the intensity of the line at 589.6 nm. Sodium Orbital Diagram. Posted on December 11, 2018December 11, 2018. Sponsored links. Related posts: Diagram Of Cellular Respiration. Photocell Wiring Diagram Pdf. EXAMPLE 8.2 Writing Orbital Diagrams Write the orbital diagram for sulfur and determine the number of unpaired electrons. Use the aufbau principle to write complete electron configurations and complete orbital diagrams for atoms of the following elements sodium, magnesium, aluminum...
Orbital Diagrams Chemistry Tutorial. Key Concepts. An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration (using the Aufau Principle to order the orbitals and hence...
Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below. Atomic no.
Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
Sodium (Na): How to write the Orbital Diagram, Electron. It also shows how to write the electron configuration of Sodium (Na) and the shorthand nobl aufbau diagram for oxygen. › Verified 3 days ago.
A guide for alien Species concerning Humans by a Human ​ Written in Union Standard Machine Communication Language (USMCL) by Henry Katou Vizex Machine Translator recommended for reading ​ Section 1: Common misconceptions regarding Hummans ​ I have met individuals of a grand total of 3 non-Human Species and have already discovered a plethora of astonishing misconceptions about my Species. I am a humble molecular network engineer, but I would like to offer t...
Electronic configuration of the Sodium atom. Valence electrons. Orbital diagram. Na (Sodium) is an element with position number 11 in the periodic table. Located in the III period.
A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row
Sodium Electron ConfigurationПодробнее. Orbital Diagrams and Electron Configuration - Basic Introduction - Chemistry Practice ProblemsПодробнее.
////////////// EXTREMELY IMPORTANT INFORMATION BELOW!////////////// (How Helium-4 acts has a ultracold gas, read bolded very carefully) ​ Ultracold atomic gases Superfluidity in an ultracold fermionic gas was experimentally proven by [Wolfgang Ketterle](https://en.wikipedia.org/wiki/Wolfgang_Ketterle) and his team who **observed** [**quantum vortices**](https://en.wikipedia.org/wiki/Quantum_vortex) in [6Li](https://en.wikipedia.org/wiki/Lithium-6) at a temperature of 50 nK at [MIT...
Neon electron configuration is 1s 2 2s 2 2p 6.The symbol for neon is ‘Ne’ and it is an inert element. This article gives an idea about the electron configuration of neon(Ne) and orbital diagram, period and groups, valency and valence electrons of neon, application of different principles. The tenth element in the periodic table is neon.
So I need someone to check some of these, so I might crosspost this to other subreddits, if you know any, please do. Or if you are an expert yourself, please correct me if there's any mistakes. But I did watch Dr. Stone in an *Anime Streaming website*, I posted some interesting comments in the discussions of Dr. Stone Episodes. I will post them in a chronological order with the matching episodes. Although, I think it's a bad idea to post this in a whole one post. Because no one gonna read it t...
Orbital Diagram. Electronic Configuration The Atom Siyavula. Steve S Drawing Illustrating The Structure Of Sodium Chloride. Sulfur Orbital Notation. Orbital Diagram Sodium Quantum Theory Hund S Rule Aufbau Principle. Atomic Orbital Filling Order Ppt Video Online Download.
Video for How To Find Orbital Diagram Molecular Orbital Diagram (MO Diagram) of Li2 Orbital Diagrams: Sodium
The most common compound of sodium is sodium chloride (common salt). It is added to food and used to de-ice roads in winter. Sodium is important for many different functions of the human body. For example, it helps cells to transmit nerve signals and regulate water levels in tissues and blood.
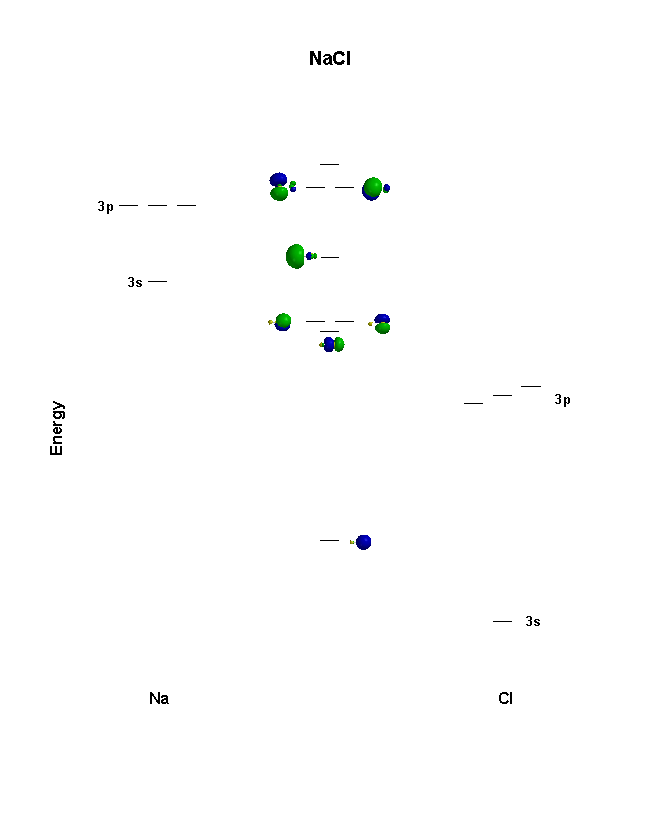
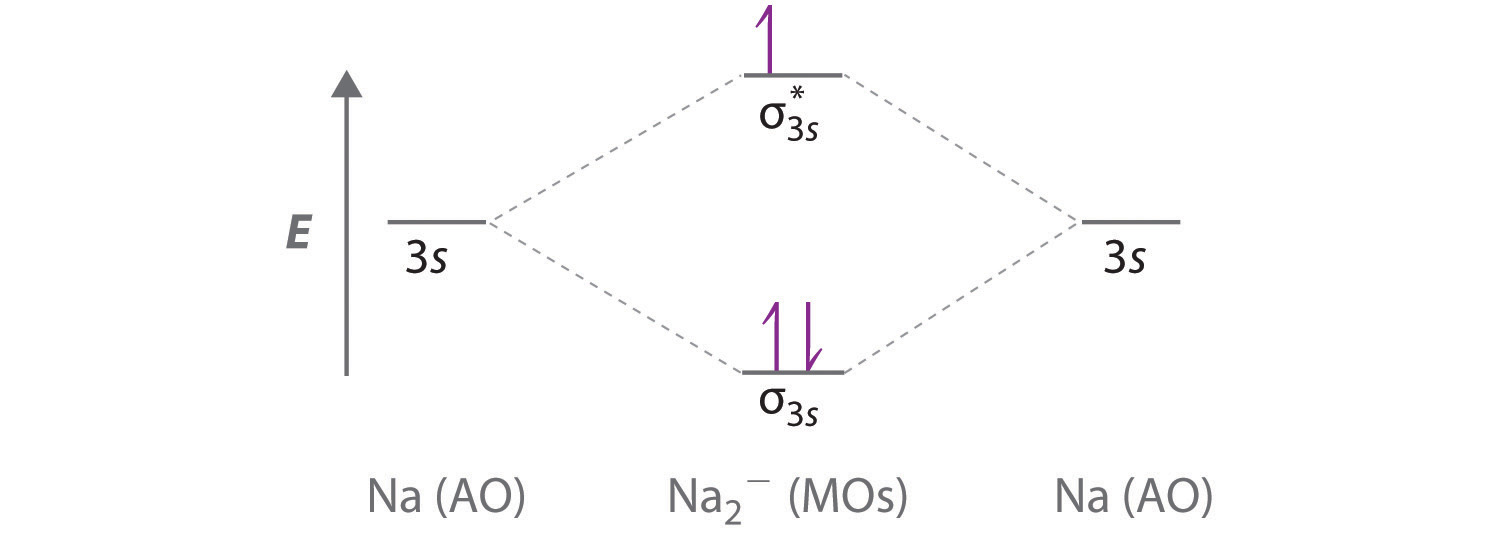
Consider the diagram of Sodium below: Circled are orbitals both containing $2$ electrons each which combine using LCAO to give a set of bonding and antibonding electrons. However, the $4$ electrons involved in this bonding appear to only give $3$ electrons in the bonding and antibonding MO's...
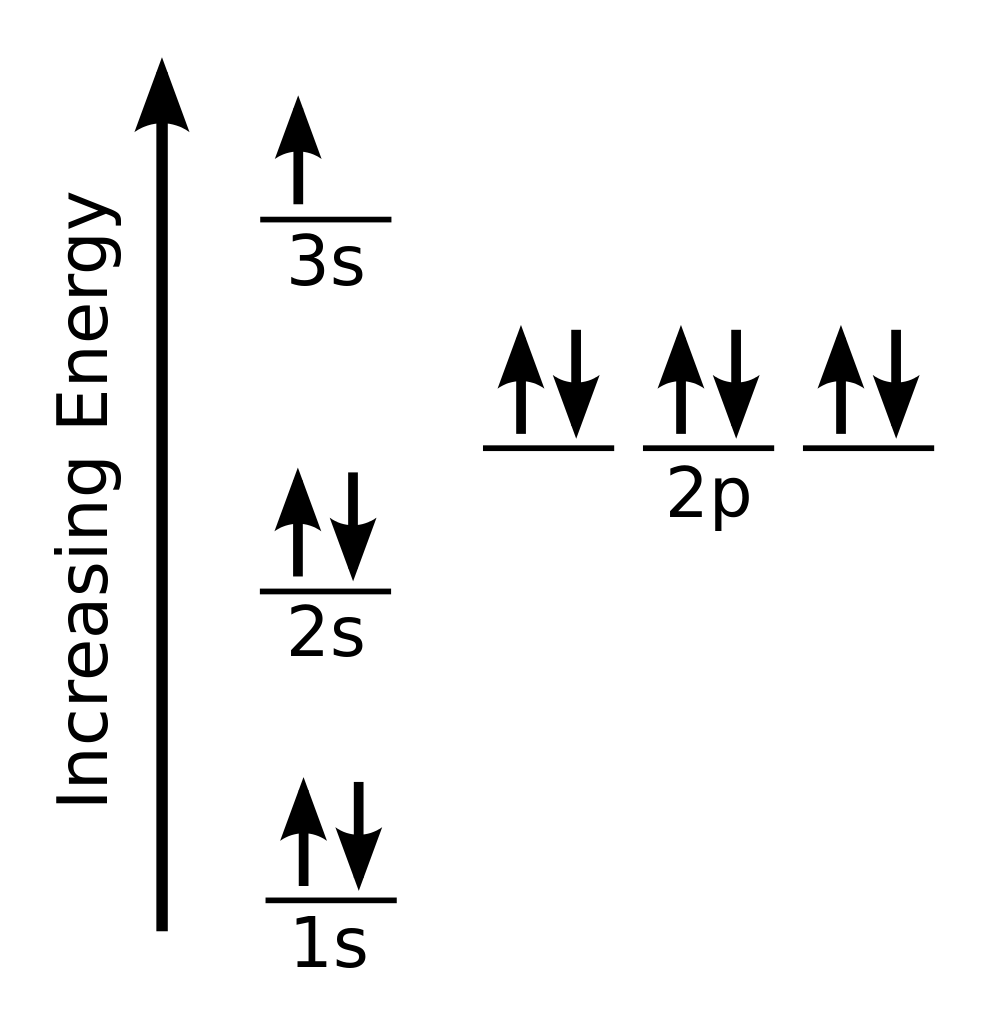
Just Now The orbital diagram of the sodium atom is shown below. The sodium atom has 11 electrons which are contained in 1s, 2s, 2p and 3s 6 hours ago 1 thought on " Electron Configuration and Orbital Box Diagram for First Excited State of Sodium " Habib Alkhaldi December 10, 2014 at 2...
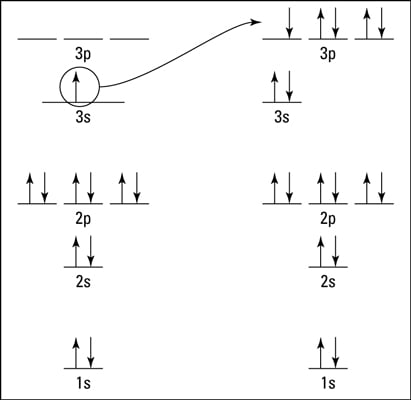
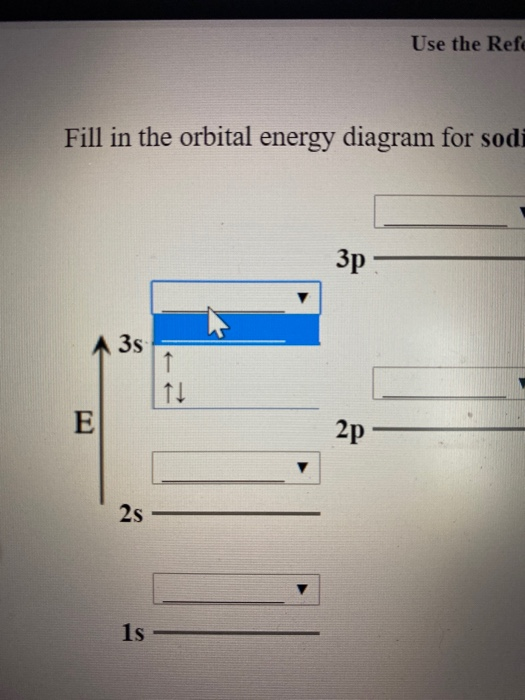
Transcribed image text: Use the Fill in the orbital energy diagram for sodium 3p 2s 1s Submit Answer Try Another Version 2 item attempts remaining.
Which of the following combination is safe as antianginal drug -OLDeltiazem+B BlockerNifedipine-B BlockerHydarlazine -Sodium nitroprussideVerapamil+BB … locker.
I'm typing this with electrodes attached to my head, 2.0 mA coursing through my neurons. I had been lurking here for a few months, trying to decide if tDCS was a worthwhile treatment. My bupropion for the last 9 years seemed to "raise my floor" in terms of my mood, but it did nothing to increase my motivation or decrease my fatigue. I only know that, for the 5\-6 months I stayed off of it as a trial at jumpstarting it back again, I was hit with an even deeper depression than I'd perhaps ever kn...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
Sodium through argon. Potassium through krypton. Periodic arrangement and trends. Figure 13: A molecular orbital energy-level diagram showing the relative energies of the atomic orbitals of atoms A and B (1sA and 1sB) and the bonding (1σ) and antibonding (2σ) molecular orbitals they form.
So this electron is the last final electron that has that set of quantum numbers, and it's a sodium atom and there's its electron, not its configuration, but its orbital diagram.
Sodium at standard temperature and pressure is a soft silvery metal that combines with oxygen in Spin-orbit interactions involving the electron in the 3p orbital split the D line into two, at 589.0 and NaK phase diagram, showing the melting point of sodium as a function of potassium concentration.
In atomic theory and quantum mechanics, an atomic orbital is a mathematical function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus.The term atomic orbital may also refer to the physical region or space where the electron can be ...
Mar 28, 2018 · Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with What orbital do the outmost electrons of sodium fill? The M orbital, there's only 1 electron in it. Why is the size of a sodium ion less that that of a sodium atom?
(Recall from Section 5.3B that two electrons in an orbital spin in opposite directions on their axes.) Therefore, if an orbital contains two electrons, its box will contain two arrows, one pointing up and the other down. Using a box diagram, we show the electron configuration of nitrogen as: Notice that the 2p electrons are shown as
Sodium is a Group 1 element (or IA in older labelling styles). Group 1 elements are often referred to as the "alkali metals". The chemistry of sodium is dominated by Soap is generally a sodium salt of fatty acids. The importance of common salt to animal nutrition has been recognized since prehistoric times.























0 Response to "39 orbital diagram of sodium"
Post a Comment