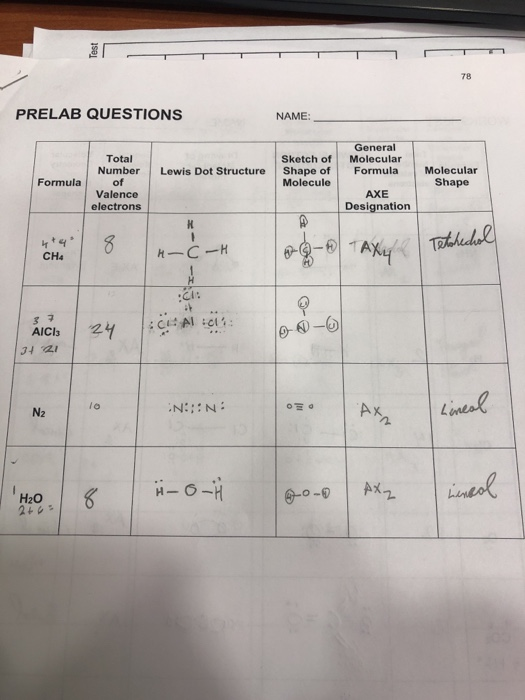
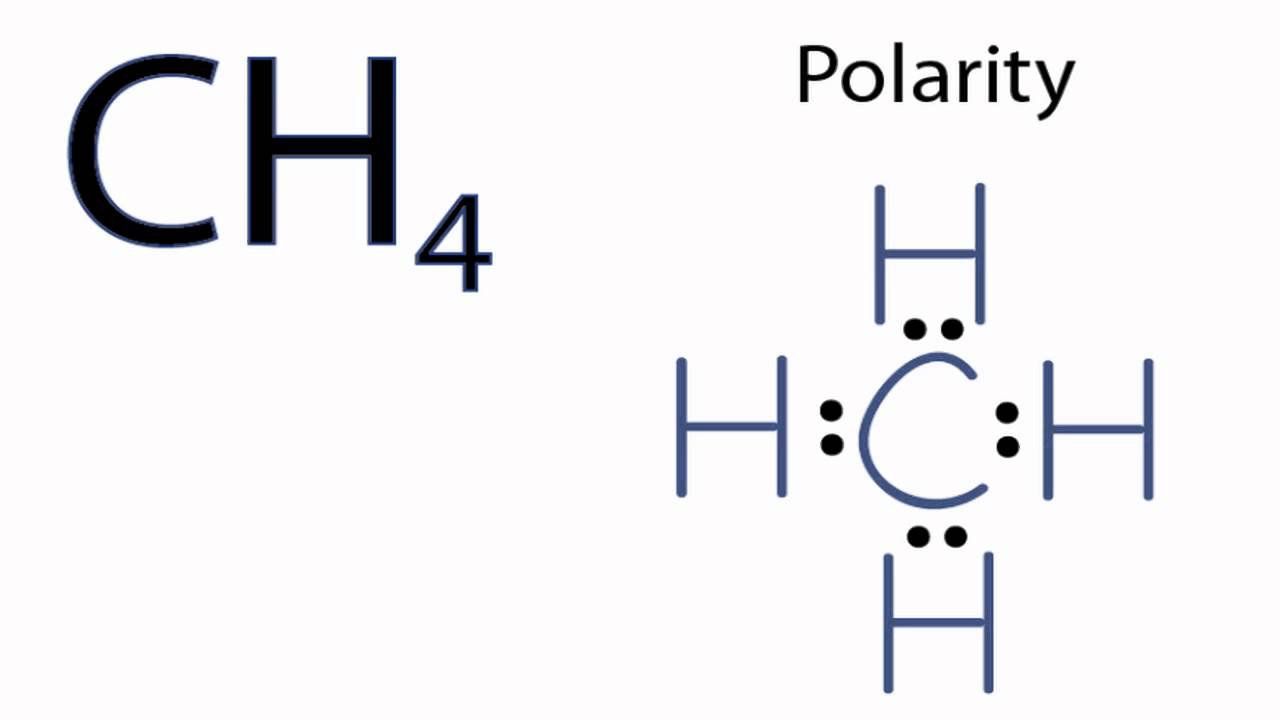
37 electron dot diagram for ch4
This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. Lewis Dot Structure for CH4 #2 Find the number of “octet” electrons for the molecule. C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 . We show two ways to draw the CH4 Lewis structure, methane. We also have This info can then be used to determine the Lewis Dot Structure.
05.11.2021 · MO Diagram of CCl4. A MO diagram is nothing but a representation of bonds that are formed within the atoms to form a compound. This diagram is based on Molecular orbital theory. With the help of a MO diagram, the existence of certain compounds can be explained. Here is the pictorial representation of how CCl4’s and CH4 MO diagram looks like.
Electron dot diagram for ch4
Draw the electron dot structure of CH4. Methane is a gas made up of one carbon atom for every four hydrogen atoms. The four hydrogen atoms around the core carbon mean that there are four groups of electrons around this carbon, giving the molecule a tetrahedral form. The core element in CH4 is carbon. The valence electron of the element is ... 07.11.2021 · Here we will learn about how the lewis dot structure is drawn for CH4 molecule, step by step. Firstly, look for the total number of valence electrons required by a single CH4 molecule, which is sixteen. Next, a search of electrons is required by a single CH4 molecule to reach a stable condition. It is eight for a single CH4 molecule, as four are needed by the carbon atom and one by hydrogen ... 1 answerMethane. The atomic number of carbon is 6. Its configuration is 2,4. Its outermost orbit contains 4 electrons. The atomic number of hydrogen is 1.
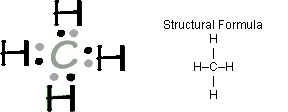
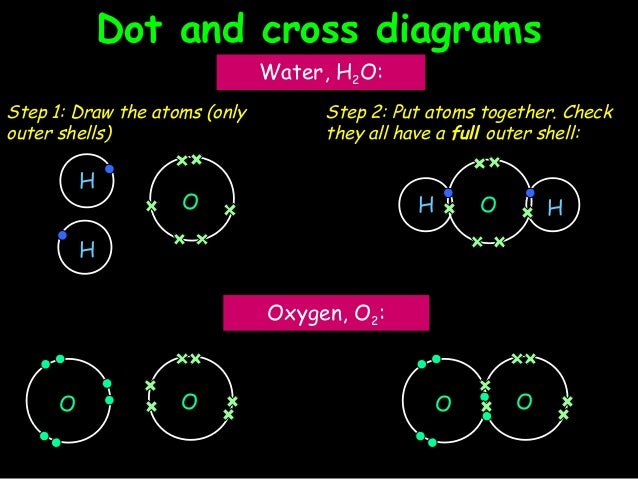
Electron dot diagram for ch4. In electron dot structure we represent the valence electron of the element. Thus, Carbon has 4 electrons in its electron dot structure and hydrogen has one. They share electrons to form a C − H single bond. Electron Dot Diagram For Methane. Well Carbon only has 4 valence electron, so it can bond at all four point. Hydrogen only has one valence electron and can only share one. Lewis symbols (also known as Lewis dot diagrams or electron dot diagrams) . Lewis dot dragram for methane: Methane, with molecular formula CH4, is shown. What is the electron dot structure for CH4? The Lewis structure of the methane (CH4) molecule is drawn with four single shared covalent bonds between the carbon and hydrogen atoms each. Moreover, as there exist sigma bonds only and one 2s and three 2p orbitals of the carbon produce four new hybrid orbitals, the hybridization of CH4 is sp3. 06.11.2021 · Lab 7 bonding in molecules and lewis structures
Hier sollte eine Beschreibung angezeigt werden, diese Seite lässt dies jedoch nicht zu. The chemical formula of methane is CH4. It is a gas that exists abundantly in nature. Now, let's know its structure: Lewis dot structure is a representation of ...1 answer · Top answer: Hint: To answer this question we should be aware of the chemical formula of methane, Lewis dot structure and the type of bond formed. This information will ... Thus, Carbon has 4 electrons in its electron dot structure and hydrogen has one. They share electrons to form a C−H single bond.1 answer · Top answer: In CH4 , the central atom is a carbon.In electron dot structure we represent the valence electron of the element.Thus, Carbon has 4 electrons in its ... Lewis dot structure of CH 4. Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule. C-4 H-1x4=4. Total=8. Put carbon in center and arrange hydrogen atoms on the diagramweb.nete electrons between carbon and hydrogen atoms. Dr.
Ch4 Electron Dot Diagram. I will explain this with pictures, and some captions. This is just the five atoms in CH4, or Methane. I have drawn them above. The red one in the. Lewis Dot Structure for CH4 #2 Find the number of “octet” electrons for the molecule. C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 . For CH4 you have a total of 8 total valence electrons. Drawing the Lewis structure for CH4 (named methane) requires only single bonds. It's one of the easier ...28 Oct 2016 · Uploaded by Wayne Breslyn Follow some steps for drawing the lewis dot structure of CF4. 1. Count total valence electron in CF4. The electrons found in the outermost shell of an atom are called valence electrons. To find the valence electrons in the CF4 molecule, look at the periodic group of each atom present in it. As carbon is situated in the 14th group and fluorine ... Practice your understanding of Lewis structures with our quiz. The quiz can give you immediate results. You can also print the quiz and finish it...
How to Draw the Lewis Dot Structure for CH4: MethaneA step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use...
Draw the electron-dot formula for SiCl2Br2. For the SiCl2Br2 Lewis structure, calculate the total number of valence electrons for the SiCl2Br2 molecule (SiCl2Br2 has 32 valence electrons). After determining how many valence electrons there are in SiCl2Br2, place them around the central atom to complete the octets. Consider thefollowing four molecules. Which of these satisfy the octet rule ...
Describe the electron dot diagram for a sodium atom. View Answer. The heats of hydrogenation for cyclohexene and benzene are given below. Calculate the resonance stabilization energy of benzene ...
18 May 2020 — The Lewis Dot Structure for CH4 is shown above. These kinds of structures can also be shown by representing each of the bonds with two dots.
Methane (CH4) lewis dot structure, molecular geometry, electron geometry, polar or nonpolar, bond angle Home > Chemistry Article > CH4 lewis structure and its molecular geometry Methane is a colorless and odorless gas formed from one atom of carbon and four atoms of hydrogen having the chemical formula CH4.
ISBN-13: 9780072534115 ISBN: 0072534117 Authors: Jeffrey A. Paradis, Jeffrey Paradis, Kristen Spotz Rent | Buy. Hands on Chemistry Laboratory Manual (1st Edition) Edit edition Solutions for Chapter 20 Problem 2: Draw the Lewis Dot Structure for methane, CH4.
The lewis diagram of an ionic compound is formed with a different approach than the normal procedure we used for drawing the compounds like NH3, BF3, BrF5, etc. Steps to draw electron dot structure or lewis dot structure of NaCl. Step 1: In the first step, we have to count the valence electron available for drawing the lewis structure of NaCl. For knowing the valence electron you have to ...
1 answerMethane. The atomic number of carbon is 6. Its configuration is 2,4. Its outermost orbit contains 4 electrons. The atomic number of hydrogen is 1.
07.11.2021 · Here we will learn about how the lewis dot structure is drawn for CH4 molecule, step by step. Firstly, look for the total number of valence electrons required by a single CH4 molecule, which is sixteen. Next, a search of electrons is required by a single CH4 molecule to reach a stable condition. It is eight for a single CH4 molecule, as four are needed by the carbon atom and one by hydrogen ...
Draw the electron dot structure of CH4. Methane is a gas made up of one carbon atom for every four hydrogen atoms. The four hydrogen atoms around the core carbon mean that there are four groups of electrons around this carbon, giving the molecule a tetrahedral form. The core element in CH4 is carbon. The valence electron of the element is ...

860-880 North Lake Shore Drive, Electrical Riser Diagram (11/28/1949) // Ludwig Mies van der Rohe (American, born Germany, 1886–1969) Associate Architect: Holsman, Holsman, Klekamp and Taylor (American, 20th century) Associate Architect: Pace Associates (American, 20th century) Structural Engineer: Frank J. Kornacker (American, active 1940s–1950s)










0 Response to "37 electron dot diagram for ch4"
Post a Comment