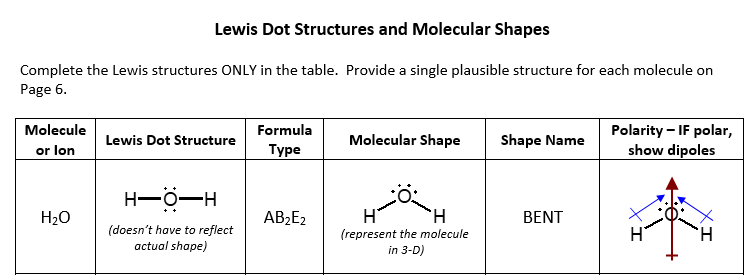
40 lewis dot diagram h2o
C2H5OH Lewis Structure - Lewis Dot Structure | Chem Helps CH3CH2OH Lewis Dot Structure is a complex structure. It's not easy to draw if you are not familiar with the topic. You should be checking H2O Lewis Structure or CO Lewis Structure first to get familiar with Lewis Structures before you try to draw CH2CH2OH Lewis Dot Structure . CH2O lewis structure, molecular geometry, bond angle ... Formaldehyde (CH2O) lewis dot structure, molecular geometry, polar or non-polar, hybridization. Formaldehyde is an organic compound that appears as a colorless gas with the chemical formula CH2O. It is the simplest aldehyde made up of two hydrogens, one carbon, and one oxygen. It is widely used as a preservative because of its antibacterial ...
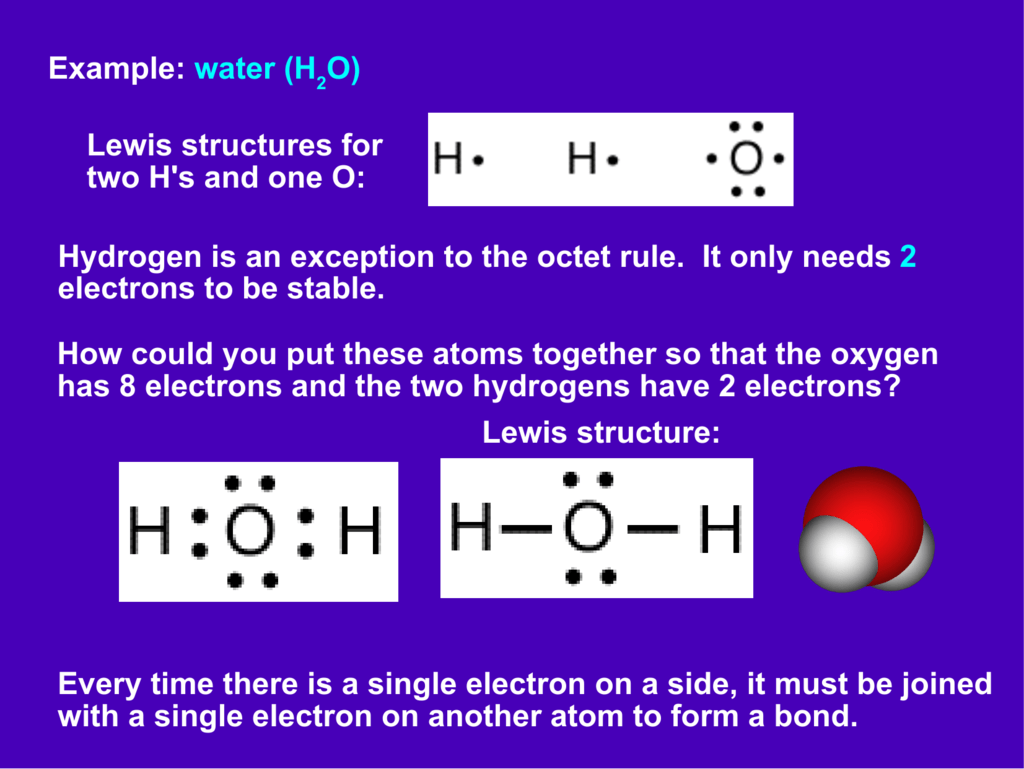
What is the Lewis structure of H2O? - Quora Originally Answered: What is a Lewis structure of H2O? A Lewis structure is a diagram that shows the bonding between the atoms of a molecule and any possible lone pairs of electrons. To ensure stability, most atoms require an octet - that is, 8 electrons in their outermost electron shell. Hydrogen, however, is an exception.
Lewis dot diagram h2o
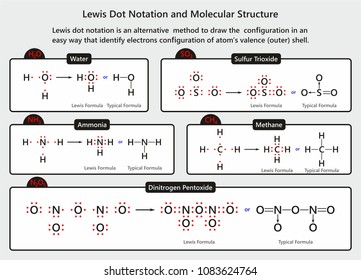
Lewis Electron Dot Structures - Detailed Explanation with ... Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the molecule. Lewis dot structures are commonly referred to as electron dot structures or Lewis structures. What is lewis dot structure for H2O? - Answers What is the Lewis dot structure for H2O? The Lewis dot structure of water begins with a single O atom in the center. On the right and left sides are a singly bonded H atom. techiescientist.com › h2o-lewis-structureH2O Lewis Structure, Molecular Geometry, and Hybridization 20.04.2022 · The Lewis structure, or also called an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom, which are ready to undergo bond formation to form a molecule and ultimately a compound. The valence electrons are shown by drawing them as dots around the symbol of the atom, mostly in pairs. …
Lewis dot diagram h2o. Lewis Dot Structure for H2O | Chemical Bonding | Success ... H 2 O Lewis Structure Video. Video Transcript: Here, we're going to do a dot structure for water, H2O. Let's write that down: H2O. What we want to find out first is how many valence electrons does water have. I'm counting all the outer shell electrons. I'll need my periodic table. H2O Molecular Geometry, Lewis Structure, Shape and Bond Angles This is the Lewis structure of the H2O molecule that has two single bonds between Oxygen and Hydrogen. As a result, there are two lone pairs in this molecule and two bonding pairs of electrons. H2O Hybridization When two atoms share electrons and form bonds, there is the formation of hybridized orbitals. patapum.to.itFlour Mill Rye [4MH368] Rye flour contains gluten, but not a lot, so it must be used in conjuction with other. 00 Quick Shop. In addition, railroads made it cheaper to ship wheat to Minneapolis/St. techiescientist.com › nocl-lewis-structureNOCl Lewis Structure, Molecular Geometry ... - Techiescientist Apr 21, 2022 · Now, all three atoms have achieved octet configurations in the above diagram. Step 6: We cannot be sure whether the sketch we have drawn is the right Lewis Structure diagram. For that, we need to check the formal charge values. In NOCl, the formal charge of N = 5 – 0.5*6 – 2 = 5 – 3 – 2 = 0.
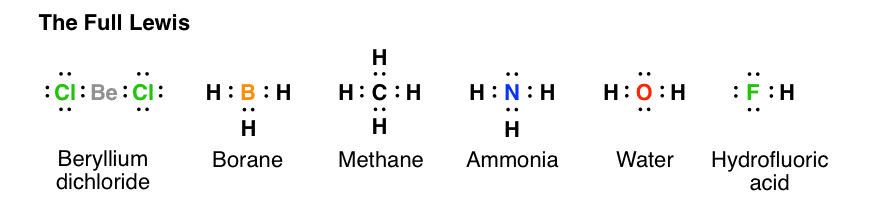
study.com › academy › lessonSingle-Displacement Reaction: Definition & Examples - Study.com Nov 05, 2021 · The Bronsted-Lowry and Lewis Definition of Acids and Bases 6:15 Neutralization and Acid-Base Reactions 5:43 Dissociation Constant and Autoionization of Water 6:41 Covalent bond and Lewis dot structure (H2O & CO2) (video ... Covalent bond and Lewis dot structure (H2O & CO2) Google Classroom Facebook Twitter. Email. Bonding in carbon- covalent bond. Carbon and hydrocarbons. Covalent bond . Covalent bond and Lewis dot structure (H2O & CO2) This is the currently selected item. Single and multiple covalent bonds. What is the Lewis dot structure of H2O? - Answers See answer (1) Best Answer. Copy. See the image of the Lewis dot structure of water in the "sources and related links" section below. Wiki User. ∙ 2012-02-27 06:19:46. This answer is: Helpful ... BeI2 Lewis Structure, Geometry, Hybridization, and ... Lewis Dot Structure of BeI2. Lewis dot structure or electron dot structure is a representation of valence electrons on an individual atom as bond pairs or lone pairs, and the position of constituent atoms of a molecule with respect to each other. In a Lewis structure, Chemical symbols represent bonding atoms. A dot represents a valence electron
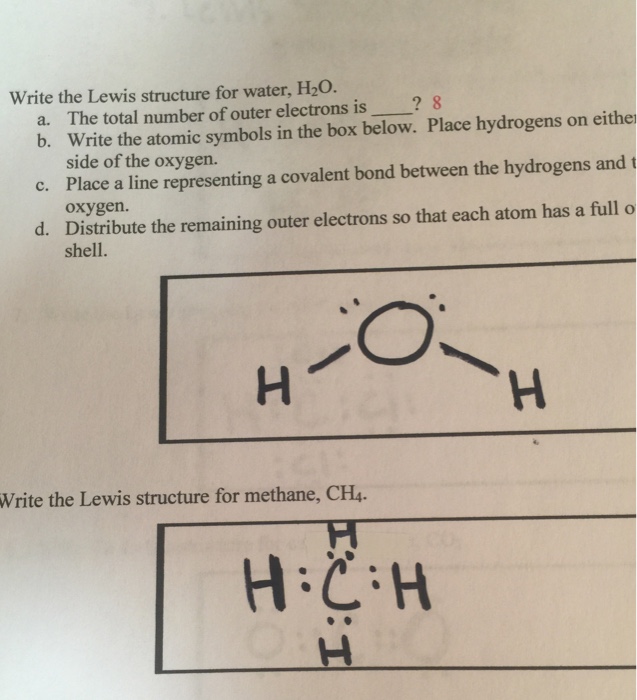
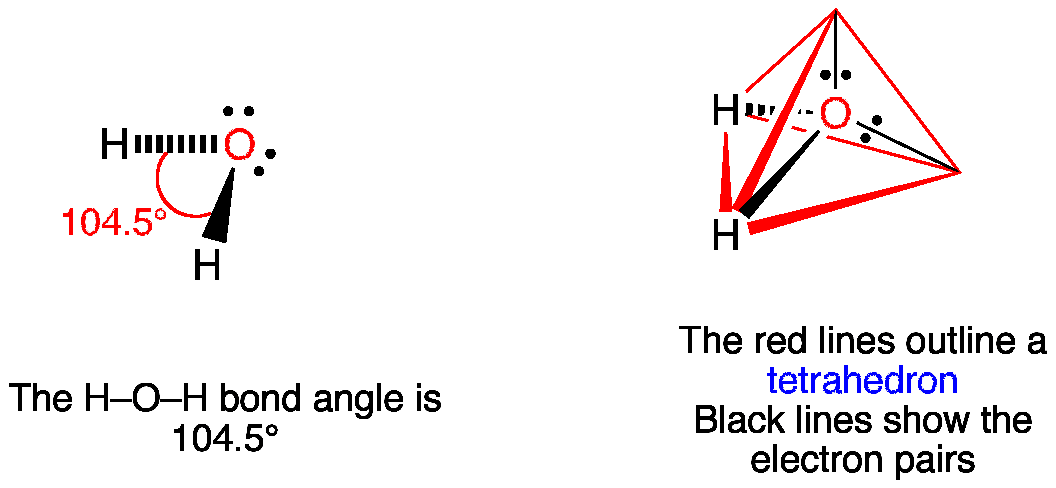
Lewis Dot Structure Answers - tsunami.as.gov more than one possible structure. Draw the best Lewis Dot Structure for each of the following species. a) BeF 2 b) BCl 3 c) CCl 4 d) PBr 5 e) SI 6. Give the name of the electronic arrangement and the name for the molecular geometry for Aug 21, 2020 · Draw the Lewis Dot Structure for CHO 2 1-and all possible resonance structures. Assign Formal ... ⚗️Two students made the Lewis dot diagrams of H2O. The ... Two students made the Lewis dot diagrams of H2O. The diagrams are as shown. Which student drew the correct Lewis dot diagram? A. Only Student A B. Only Student B C. Both Student A and Student B D. Neither Student A nor Student B Lewis Dot Diagram H2o - schematron.org This is the Lewis Dot Structure for H2O. You could alternatively also draw the structure by including two dots for every bond. While oxygen's octet seems to have.The Lewis dot structure of water begins with a single O atom in the center. On the right and left sides are a singly bonded H atom. Phase diagram of water - Columbia University Lewis dot diagram O H 104.5o H space filling model. O-H bonds are polarized because of the difference in electronegativity between the O and H atoms. Hydrogen bonds This unequal electron distribution results in strong non-bonding interactions between water molecules - hydrogen bonds. Hydrogen bonds in liquid water . Consequences of hydrogen bonding in water Ice floats …
Water Lewis Structure - How to Draw the Lewis Structure ... A step-by-step explanation of how to draw the H2O Lewis Dot Structure (Water).For the H2O structure use the periodic table to find the total number of valenc...
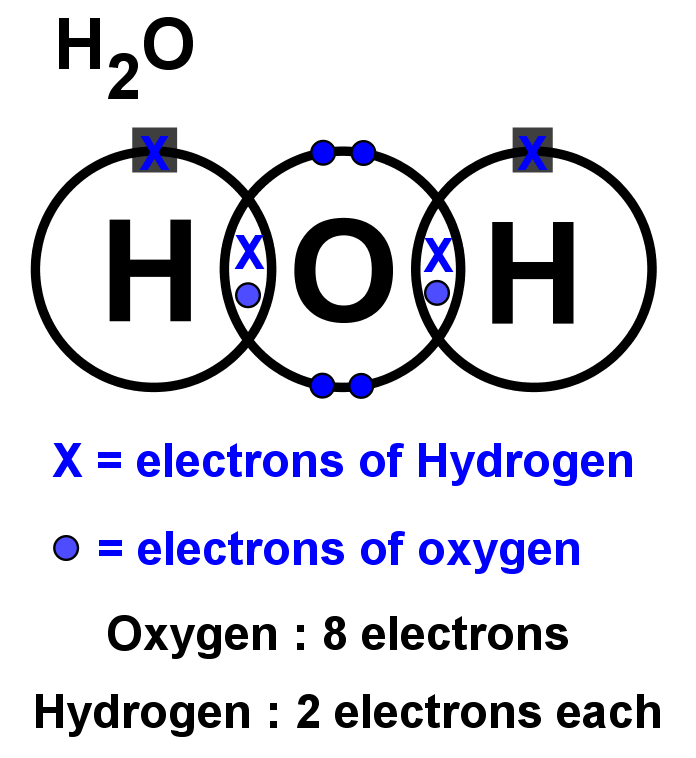
Lewis Dot Structure For H2o - Wiring Schematic Online The lewis structure of hydrogen and 2 oxygen atoms shows a total of eight valence electrons participate in the bond formation to form a single triatomic h2o molecule. On the right and left sides are a singly bonded h atom. And oxygen is in group 6 sometimes called 16 so it has 6 valence electrons. 2 plus 6 equals 8. So 1 times 2 is 2 plus 6.
Lewis acids and bases - Wikipedia A center dot may also be used to represent a Lewis adduct, such as Me 3 B·NH 3. Another example is boron trifluoride diethyl etherate, BF 3 ·Et 2 O. In a slightly different usage, the center dot is also used to represent hydrate coordination in various crystals, as in MgSO 4 ·7H 2 O for hydrated magnesium sulfate, irrespective of whether the water forms a dative bond with the …
What is the Lewis dot structure for h2o? - FindAnyAnswer.com Drawing the Lewis Structure for H2O You have a total of 8 valence electrons available to fill the octets of Oxygen and Hydrogen. Remember that Hydrogen only needs two electrons to have a full outer shell. It is helpful if you: Try to draw the H2O Lewis structure before watching the video. What is the structural formula of water? H2O
Draw Lewis dot diagram for the following. Water (H2O ... Draw Lewis dot diagram for the following. Water (H2O) Maharashtra State Board HSC Science (Computer Science) 11th. Textbook Solutions 6926. Important Solutions 17. Question Bank Solutions 4568. Concept Notes & Videos 336. Syllabus. Advertisement Remove all ads. Draw Lewis dot diagram for the following. ...
› 20389225 › Handbook_of_ChemistryFree PDF Download - Handbook-of-Chemistry-and-Physics ... Academia.edu is a platform for academics to share research papers.
(PDF) Inorganic Chemistry 4th edition ... - Academia.edu Inorganic Chemistry 4th edition, Catherine Housecroft
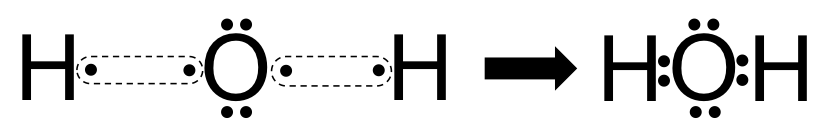
How can I draw the Lewis structure for H2O? | Socratic 1 Answer. Ernest Z. Jul 15, 2014. You can find a procedure for drawing Lewis structures at this location. For H₂O, O must be the central atom. The skeleton structure is H-O-H. O has 6 valence electrons, and each H has one. You must arrange 8 electrons in pairs so that O has 8 and each H has two electrons in its valence shell.
CH3CH2CH3 Lewis Structure - Lewis Dot Structure | Chem Helps Because C has 4 valence electrons and H has 1 valence electron. 4×3 + 8×1 = 20 valence electrons. Since we know valence electrons we can find the total electron pairs of CH3CH2CH3 molecule. We need to divide total valence electrons by 2. 20/2 = 10. CH3CH2CH3 has 10 total electron pairs. Center atom should be Carbon There are no lone pairs.
Lewis Structure of H2O (Water) - Drawing Steps In the lewis structure of H 2 O, there are two single bonds around oxygen atom. Hydrogen atoms are joint to oxygen atom through single bonds. Also, there are two lone pairs on oxygen atom. Water molecule is a simple molecule. Drawing lewis structure of water molecule is simple than some of other complex molecules or ions.
Lewis Dot Structure of H2O, (Water) - YouTube I quickly take you through how to draw the Lewis Structure of water, H2O. I also go over hybridization, shape and bond angle.
pubs.acs.org › doi › 10Solar Water Splitting Cells | Chemical Reviews Nov 10, 2010 · The dot-dashed line, however, shows NiCo 2 O 4 with respect to projected, rather than active surface area, demonstrating that the apparent activity of a catalyst with a high roughness factor increases dramatically if one does not account for the catalytic activity with respect to the active surface area.
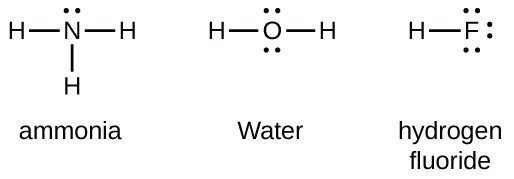
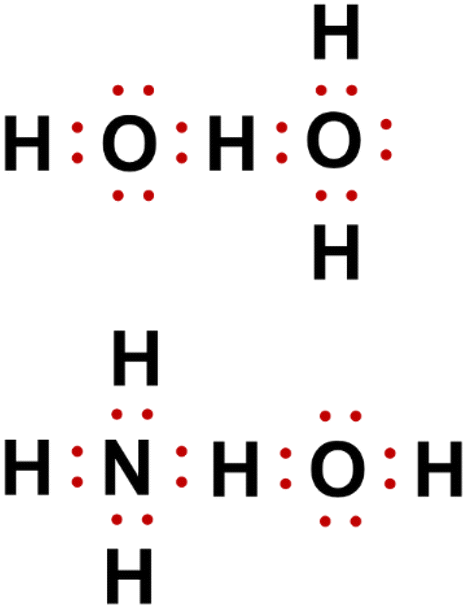
Lewis dot diagrams for the following:(a) Hydrogen (H2) (b ... Click here👆to get an answer to your question ️ Lewis dot diagrams for the following:(a) Hydrogen (H2) (b) Water (H2O) (c) Carbon dioxide (CO2) (d) Methane (CH4)
How do you draw a Lewis dot structure for water? Answer and Explanation: Water, or H2O H 2 O , has the electron dot structure shown below. The structure must have a total of 8 valence electrons because there are 2 Also, what type of bond is water? Water is a polar molecule A water molecule is formed when two atoms of hydrogen bond covalently with an atom of oxygen.
MakeTheBrainHappy: The Lewis Dot Structure for H2O H2O's Lewis Dot Structure gives it many unique properties mostly due to the two lone pairs on the central oxygen atom. This increases electron-electron repulsion and therefore creates a bent structure as opposed to CO2's linear structure. This "bent" molecular structure gives it many unique properties such as being polar.
H2o2 Lewis Dot - lewis dot diagram for ccl4 free wiring ... H2o2 Lewis Dot. Here are a number of highest rated H2o2 Lewis Dot pictures on internet. We identified it from obedient source. Its submitted by organization in the best field. We allow this kind of H2o2 Lewis Dot graphic could possibly be the most trending topic considering we allowance it in google lead or facebook.
Lewis Dot Symbols and Lewis Structures | Boundless Chemistry The former, known as a 'Lewis dot diagram,' indicates a pair of shared electrons between the atomic symbols, while the latter, known as a 'Lewis structure,' uses a dash to indicate the pair of shared electrons that form a covalent bond. More complicated molecules are depicted this way as well.
Lewis Dot Diagram H2o The Lewis dot structure for water shows the electron from hydrogen and an electron from oxygen being shared in a covalent bond. The other four valence electrons in oxygen are in pairs at the bottom. The Lewis dot structure of water begins with a single O atom in the center. On the right and left sides are a singly bonded H atom.
techiescientist.com › h2o-lewis-structureH2O Lewis Structure, Molecular Geometry, and Hybridization 20.04.2022 · The Lewis structure, or also called an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom, which are ready to undergo bond formation to form a molecule and ultimately a compound. The valence electrons are shown by drawing them as dots around the symbol of the atom, mostly in pairs. …
What is lewis dot structure for H2O? - Answers What is the Lewis dot structure for H2O? The Lewis dot structure of water begins with a single O atom in the center. On the right and left sides are a singly bonded H atom.
Lewis Electron Dot Structures - Detailed Explanation with ... Lewis dot structures also called electron dot structures are diagrams that describe the chemical bonding between atoms in a molecule. They also display the total number of lone pairs present in each of the atoms that constitute the molecule. Lewis dot structures are commonly referred to as electron dot structures or Lewis structures.


/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)


/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)










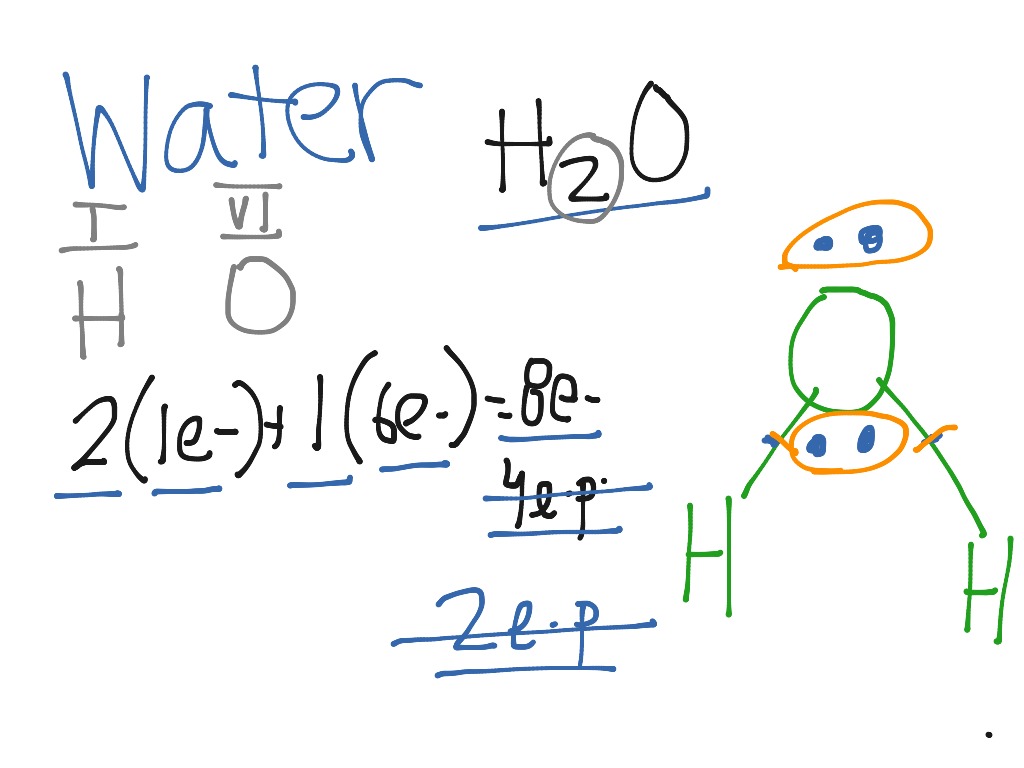

:max_bytes(150000):strip_icc()/ICl3_LD-56a12a2b3df78cf77268034c.png)


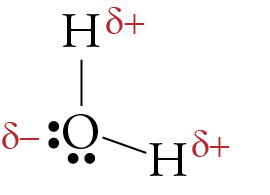




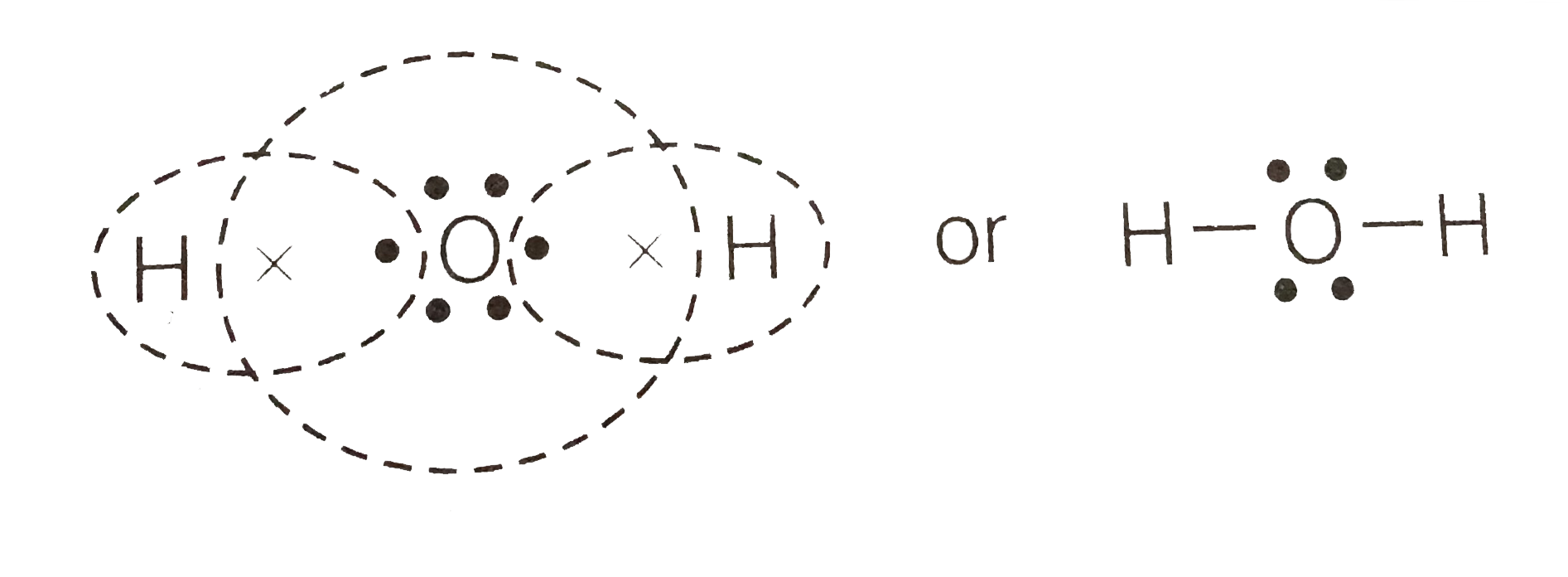
0 Response to "40 lewis dot diagram h2o"
Post a Comment