38 crystal field splitting diagram
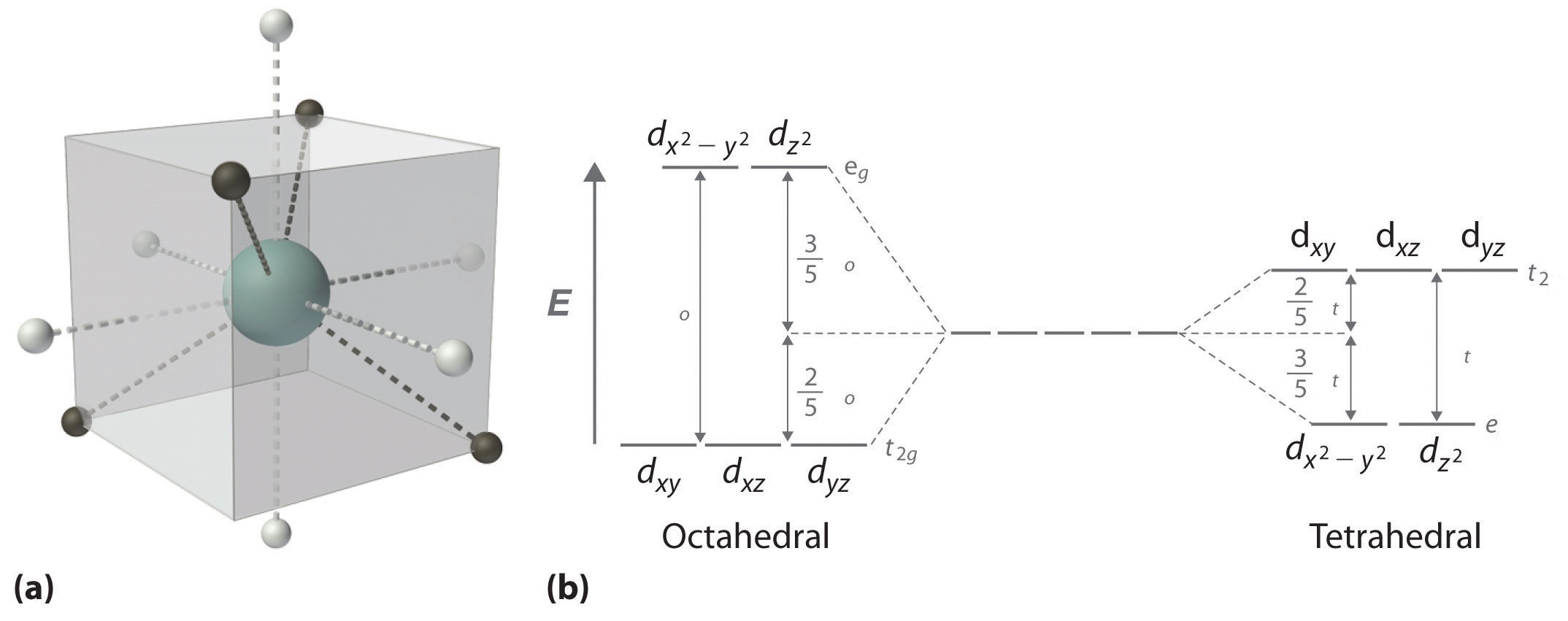
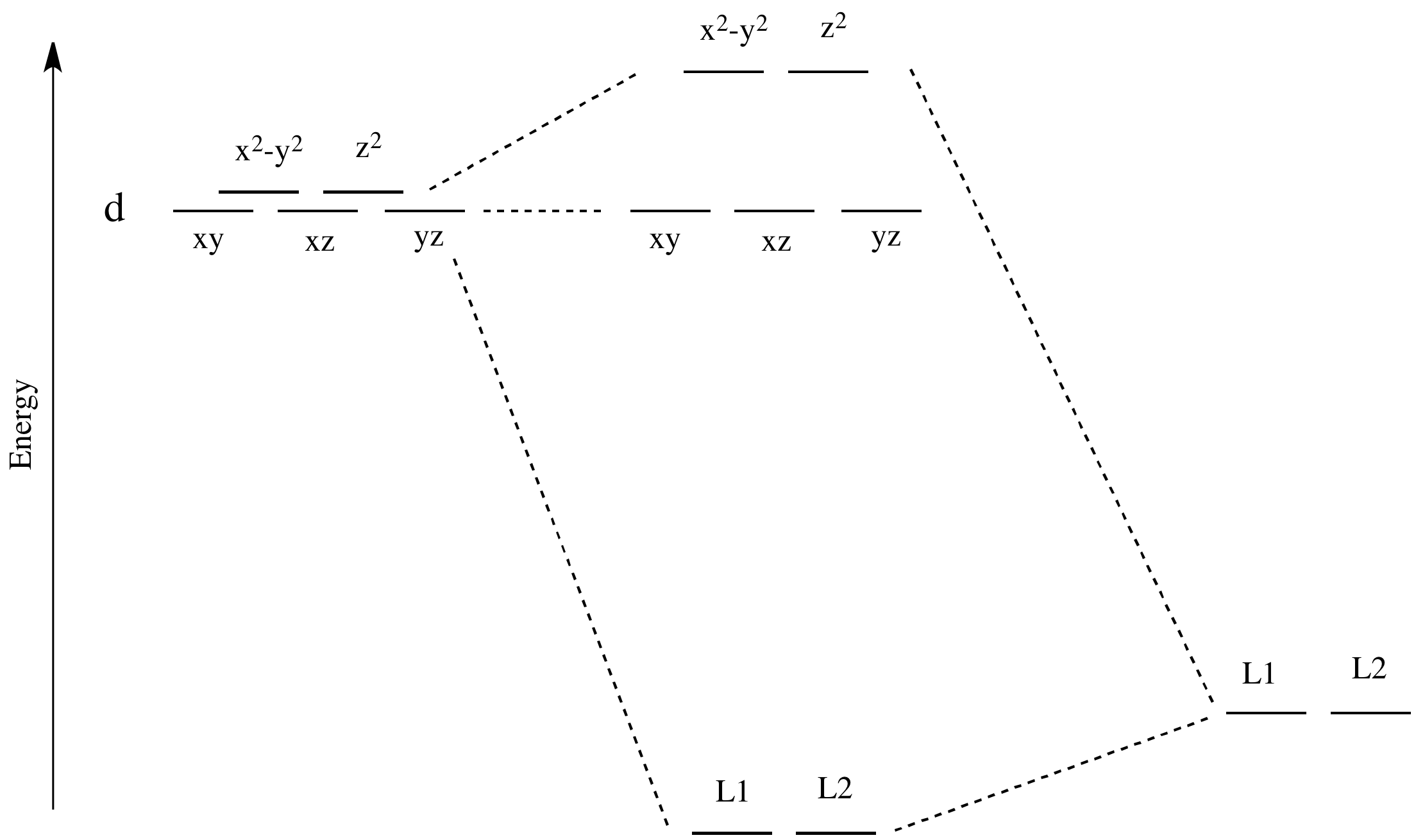
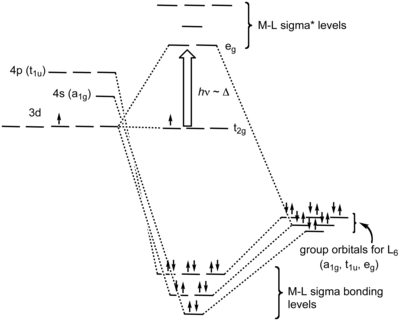
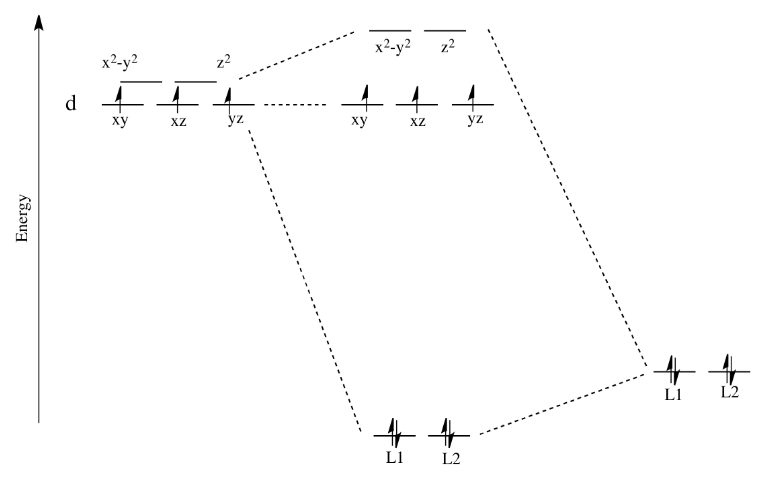
Crystal Field Theory (CFT) - Detailed Explanation with ... Crystal Field Splitting in Tetrahedral Complex The splitting of fivefold degenerate d orbitals of the metal ion into two levels in a tetrahedral crystal field is the representation of two sets of orbitals as T d. The electrons in d x2-y2 and d z2 orbitals are less repelled by the ligands than the electrons present in d xy, d yz, and d xz orbitals. Crystal Field Theory | Definition, Examples, Diagrams Crystal field theory. The crystal field theory (CFT) is an electrostatic model which considers the metal-ligand bond to be ionic arising purely from electrostatic interactions between the metal ion and the ligand. Ligands are treated as point charges in case of anions or dipoles in case of neutral molecules.
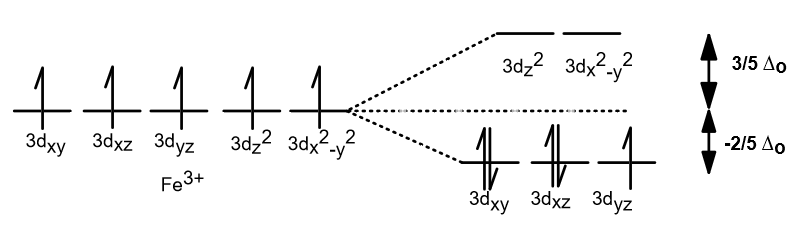
Construct The Octahedral Crystal-field Splitting Diagram ... Construct the octahedral crystal-field splitting diagram for the metal in each species. Cr4+ Mn (H2O)6^2+. Nov 14, · Basically, the question is referring to the compound K3 [Fe (C2O4)3]. It asks what is the electron configuration in this comound, I got it to be d5. Fe in the compound is Fe (III) so 23 electrons -> d5.

Crystal field splitting diagram
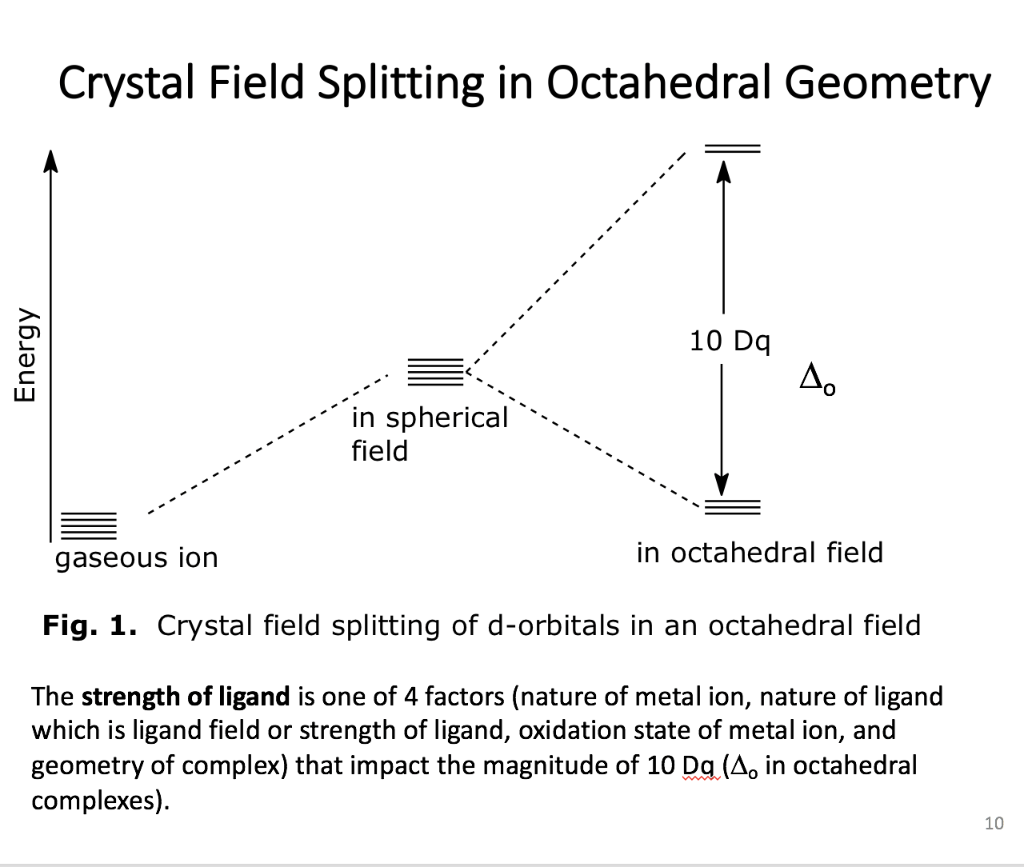
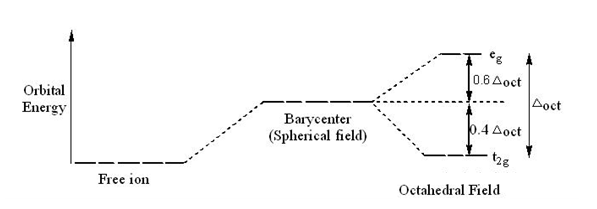
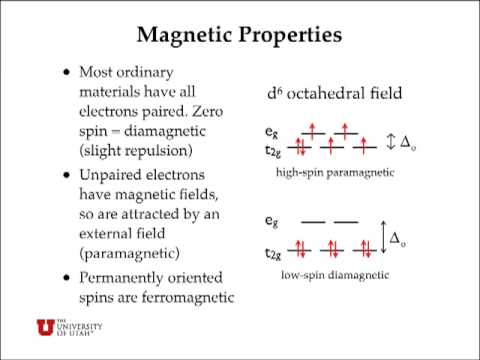
Why is "Ni"("CN")_4^(2-) diamagnetic but "NiCl"_4^(2 ... CRYSTAL FIELD SPLITTING DIAGRAMS. Their blank d -splitting diagrams within the realm of crystal field theory are: [Ni(CN)4]2−: The d orbitals fill with 8 electrons, then, with a low spin configuration. You can see that an even number of d orbitals will get filled ( dyz,dxz,dz2,dxy) with an even number of 3d electrons. Draw figure to show the splitting of d ... - SaralStudy Question 16 Draw figure to show the splitting of d orbitals in an octahedral crystal field. Answer The splitting of the d orbitals in an octahedral field takes palce in such a way that d x2y2 , d z2 experience a rise in energy and form the eg level, while d xy, d yz and d zx experience a fall in energy and form the t 2g level. Crystal Field Theory - YouTube This chemistry video tutorial provides a basic introduction into crystal field theory. It explains how to draw the crystal field splitting diagram of transi...
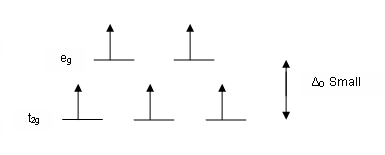
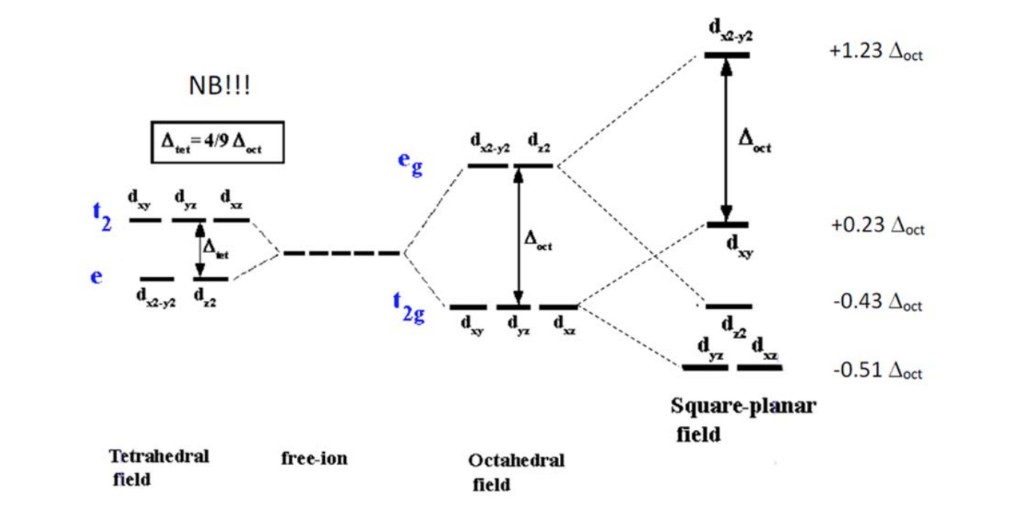
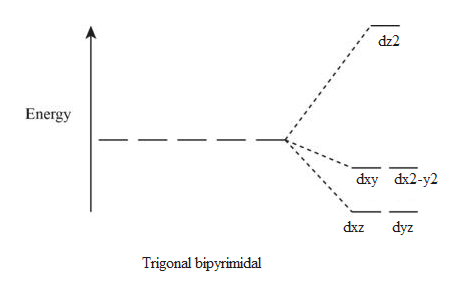
Crystal field splitting diagram. (B) Draw the crystal field splitting diagram for the above ... In a coordination entity, the electronic configuration of the central metal ion is t2g3eg1(B) Draw the crystal field splitting diagram for the above complex.Answer(B) Square Planar D Orbital Splitting Diagram We find that the square planar complexes have the greatest crystal field splitting ligand field (left diagram) and the tetrahedral field (right diagram).D-orbital splitting diagrams Use crystal field theory to generate splitting diagrams of the d-orbitals for metal complexes with the following coordination patterns: 1. Octahedral 2. Tetrahedral 3. Welcome to Chem Zipper.com......: CRYSTAL FIELD SPLITTING ... (1) There are only four ligands instead of six, so the ligand field is only two thirds the size; as the ligand field spliting is also the two thirds the size and (2) The direction of the orbitals does not concide with the direction of the ligands. This reduces the crystal field spliting by roughly further two third. Crystal Field Splitting - an overview | ScienceDirect Topics Crystal field d orbital splitting diagrams for common geometries. The above treatment considers the ligands in an octahedral geometry (i.e., with the ligands placed at the centre of the faces of the cube). The square planar case is simply a special case of the octahedral symmetry where two ligands are removed from the z -axis.
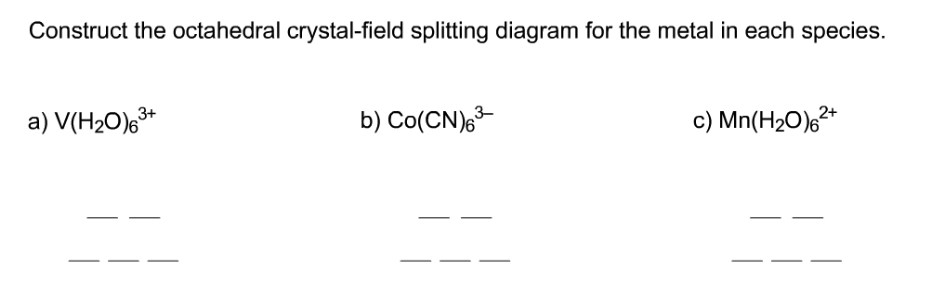
Construct The Octahedral Crystal-field Splitting Diagram Answer to Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)63+ Co (CN)63 - Mn (H2O)62+. A d1 octahedral complex is found to absorb visible light, with the absorption maximum occcurring at nm. a) Calculate the crystal-field splitting energy, Δ, in. PDF Comparative Crystal Field Studies of Some Ligand of Cr3 ... Crystal Field Splitting Parameter (Δ o) or, 10 D q. The usage of a Tanabe-Sugano (TS) diagram in spectral investigation is reasonably simple. Figure 1 shows a basic Tanabe-Sugano diagram for a d3 4metal ion. Transitions between the A 2g ground state and excited states, possessing the similar multiplicity, can be characterized as perpendicular ... Crystal Field Theory - YouTube This chemistry video tutorial provides a basic introduction into crystal field theory. It explains how to draw the crystal field splitting diagram of transi... Draw figure to show the splitting of d ... - SaralStudy Question 16 Draw figure to show the splitting of d orbitals in an octahedral crystal field. Answer The splitting of the d orbitals in an octahedral field takes palce in such a way that d x2y2 , d z2 experience a rise in energy and form the eg level, while d xy, d yz and d zx experience a fall in energy and form the t 2g level.
Why is "Ni"("CN")_4^(2-) diamagnetic but "NiCl"_4^(2 ... CRYSTAL FIELD SPLITTING DIAGRAMS. Their blank d -splitting diagrams within the realm of crystal field theory are: [Ni(CN)4]2−: The d orbitals fill with 8 electrons, then, with a low spin configuration. You can see that an even number of d orbitals will get filled ( dyz,dxz,dz2,dxy) with an even number of 3d electrons.










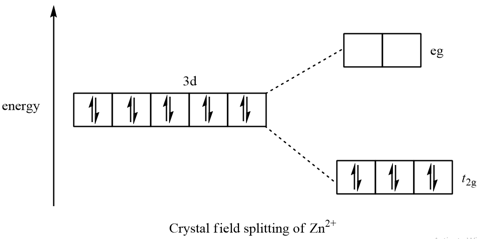










0 Response to "38 crystal field splitting diagram"
Post a Comment