41 solid liquid phase diagram
PDF Binary Solid-Liquid Phase Diagram Introduction remaining liquid solution. In the salt water analogy, the solid ice (pure H2O) is in equilibrium with the liquid H2O that remains in the unfrozen salt water. Mixtures of naphthalene and diphenylamine, both solids in the pure state at room temperature, will be prepared and their phase transitions studied by means of a thermal analysis. Phase Diagrams | Boundless Chemistry - Lumen Learning Phase Diagram: In this phase diagram, which is typical of most substances, the solid lines represent the phase boundaries.The green line marks the freezing point (or transition from liquid to solid), the blue line marks the boiling point (or transition from liquid to gas), and the red line shows the conditions under which a solid can be converted directly to a gas (and vice-versa).
Phase Diagrams | Liquids and Solids - Nigerian Scholars A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures ...
Solid liquid phase diagram
Solid-liquid phase equilibrium and phase diagram of the ... The phase diagram belongs to a simple-type ternary system, and neither double salt nor solid solution was formed. Based on the phase diagrams of this system at 298.15 and 338.15 K, the cycle separation process for CsNO 3 recovery from the eluant of sodium nitrate mixture solution was evolved. Solid-Liquid Phase Diagrams - YouTube Describes the regions of a liquid-solid, T-x phase diagram for a system composed of Mg and Si. Made by faculty at the University of Colorado Boulder Departme... Solid-Solid-Liquid Phase Diagram (Interactive Simulation ... Describes an interactive simulation that shows the phase diagram (temperature versus mole fraction) for two solids that form a solid compound. Heat can be ad...
Solid liquid phase diagram. How to figure out which phase on a phase diagram will be ... Usually the solid phase is the densest. To be sure, look at the slope of the solid-liquid line. If it is decreasing, the liquid phase is denser, if it is increasing, the solid phase is denser. For example, the phase diagram of water has a negative solid-liquid line; the liquid phase of water is denser. 10.4 Phase Diagrams - Chemistry Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ... Liquid and Solid Solution phase changes - First Year ... The solid/liquid solution phase diagram can be quite simple in some cases and quite complicated in others. Let's begin by looking at a simple two-component phase diagram with components that are fully miscible in both the liquid and solid phase. The diagram to the right shows a simple two-component A,B system solid-liquid phase diagram. In the phase diagram for water, indicate the | Chegg.com Science. Chemistry. Chemistry questions and answers. In the phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move along the temperature axis after the addition of solute, Aner Bank 1 liquid Pressure (atm) solid gas Temperature ("C)
PDF Experiment 1 Solid - Liquid Phase Diagram SOLID - LIQUID PHASE DIAGRAM Important: bring a formatted 3.5" floppy diskette/USB flash drive for this laboratory - you will need it to save your data files! Introduction The relation of cooling curves to phase diagrams form the basis of "thermal analysis", an important technique for constructing phase diagrams . Solid-liquid Phase Diagrams: Tin and Lead solid-liquid phase diagrams: tin and lead This page explains the relationship between the cooling curves for liquid mixtures of tin and lead, and the resulting phase diagram. It also offers a simple introduction to the idea of a eutectic mixture. PDF Determination of the Solid-Liquid Phase Diagram for ... • Phase Diagrams Phase diagrams are graphs that give information on the equilibrium temperature and pressure for a particular compound. The equilibria occur for the solid- liquid plateau, liquid-vapor plateau and solid-vapor plateau. In this experiment, the phase diagram is shown for the solid-liquid equilibrium point, and varies from 100% ... 1.14 Phase Diagrams Quiz Flashcards | Quizlet For a given sample of carbon dioxide (CO2) you increase the temperature from −80°C to 0°C and decrease the pressure from 30 atm to 5 atm . On a phase diagram this causes a crossing of both the solid-liquid boundary and the liquid-gas boundary.
Phase Diagram for Water | Chemistry for Non-Majors Notice one key difference between the general phase diagram and the phase diagram for water. In water's diagram, the slope of the line between the solid and liquid states is negative rather than positive. The reason is that water is an unusual substance in that its solid state is less dense than the liquid state. Ice floats in liquid water. Liquid-Solid Phase Diagrams: Tin and Lead - Chemistry ... If you cooled a liquid mixture on the right-hand side of the phase diagram (to the right of the eutectic mixture), everything would work exactly the same except that solid tin would be formed instead of solid lead. PDF Liquid-Solid Phase Diagrams - Texas A&M University Liquid-Solid Phase Diagrams Solid and liquid phases can be present below the boiling point (e.g., immiscible pair of metals right up to their melting points (As and Bi) 2-component liquid at temperature a1: (1) a1 6 a2 System enters "Liquid+B" pure solid B comes out of solution, remaining liquid richer in A (2) a2 6 a3 More solid B forms, equal Solid Solution Phase Diagram - James Madison University The solid solution phase diagram explains the behavior of chemical solid solution series, such as the transition from high temperature, calcium-rich plagioclase to low temperature sodium-rich plagioclase, or the transition from high temperature magnesium-rich to low temperature iron-rich crystals in ferromagnesium minerals (e.g. olivine, pyroxene).
Chapter 8 Phase Diagrams - Central Michigan University The system enters the two phase region labeled 'liquid + B'. Pure solid B begins to come out of solution and the remaining liquid becomes richer in A. (2) a 2 ® a 3. More of the solid forms, and the relative amounts of the solid and liquid (which are in equilibrium) are given by the lever rule.
Solid-liquid Phase Diagram | USP Technologies Solid-liquid Phase Diagram . Ref: P.A. Giguere. "Complements au Nouveau Traite de C. himie Minerale - No. 4 - Peroxyde d'Hydrogene et Polyoxydes d'Hydrogene" Paris, Masson 1975 (181 p).
Phase Diagrams - Chemistry A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures ...
PDF Phase Diagrams, Solid Solutions, Phase Transformations •The phase diagram plots relative concentrations of A and B along the X-axis, and temperature along the Y-axis. The eutectic point is the point where the liquid phase borders directly on the solid α + β phase; it represents the minimum melting temperature of any possible A B alloy.
PDF Simple Solid - Liquid Phase Equilibria #4 A fundamental problem of interest in Materials Science is the calculation of solid - liquid phase diagrams. Many systems of similar compounds form simple eutectic solutions. The phase diagram for such a system and the equations that describe the phase transition lines are shown in our class notes.
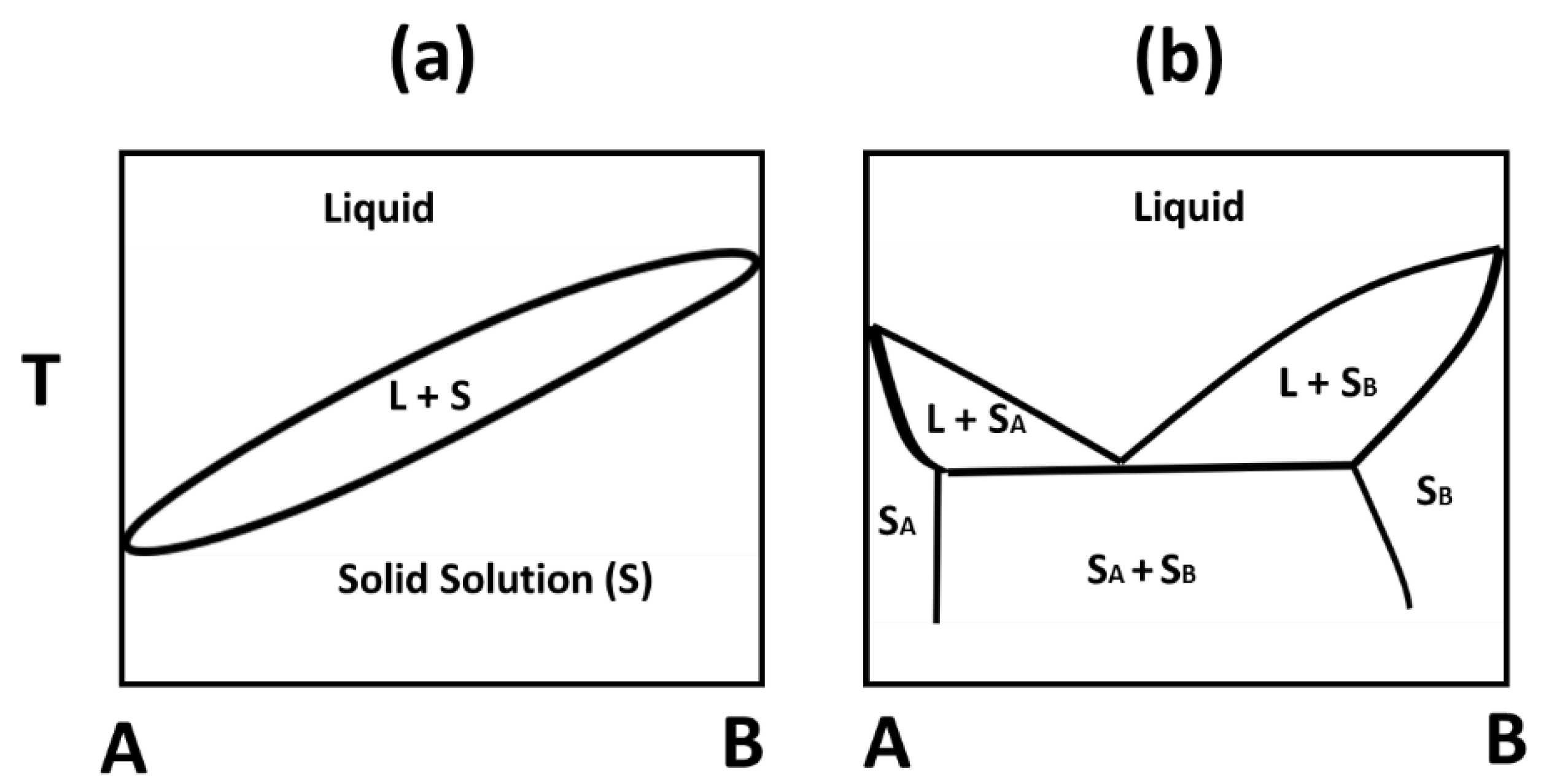
Solid-Liquid Equilibrium - an overview | ScienceDirect Topics The components of a solid solution completely dissolve in one another in a manner similar to liquids forming homogeneous solution. As a result, the system does not have a eutectic point and the solid-liquid phase diagram shown in Fig. 12.4 resembles the temperature-composition diagram for a vapor-liquid mixture, i.e. Fig. 9.3. The two-phase region in the middle is bounded by the liquidus and ...
PDF Chapter Outline: Phase Diagrams ¾Solidification in the solid + liquid phase occurs gradually upon cooling from the liquidus line. ¾The composition of the solid and the liquid change gradually during cooling (as can be determined by the tie-line method.) ¾Nuclei of the solid phase form and they grow to consume all the liquid at the solidus line.
Solid Liquid Phase Diagram - Solid-Liquid Phase Diagram in ... Solid-Liquid Phase Diagram in a Two-Component System 1 Introduction The substances we encounter everyday are commonly mixtures of two or more components. For example, brass is a mixture of copper and zinc, and dish-washing detergent is a mixture of many chemicals. The components may interact with each other in a variety of different manners. In this experiment, you will investigate a simple ...
Answered: INTERPRETING PHASE DIAGRAMS OF WATER… | bartleby INTERPRETING PHASE DIAGRAMS OF WATER AND CARBON DIOXIDE 218 Liquid B Liquid Solid Solid 5.2 Gas Gas 0.006 273 273.2 373 647 197.5 216.6 304.25 Phase diagram of water Phase diagram of CO, Based from the phase diagrams of water and carbon dioxide, answer the following questions and justify your answers: 1.
Binary Solid-Liquid Phase Diagram | Chem Lab Introduction. Solid-liquid phase diagrams show the phase relationships in mixtures of two or more components and are very important in understanding the behavior of mixtures in metallurgy, material science and geology. In this exercise, you will measure cooling curves of either the napthalene-biphenyl system (group 1) or the napthalene-durene ...
PDF Chapter 9: Phase Diagrams - Florida International University • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash (Ed.), ASM International, Materials Park, OH (1991). • 2 phases: L (liquid) α (FCC solid solution) • 3 phase fields: L L + α α 0 20 40 60 80 100 wt% Ni 1000 1100 1200 1300 1400 1500 1600 ...
Solid-Solid-Liquid Phase Diagram (Interactive Simulation ... Describes an interactive simulation that shows the phase diagram (temperature versus mole fraction) for two solids that form a solid compound. Heat can be ad...
Solid-Liquid Phase Diagrams - YouTube Describes the regions of a liquid-solid, T-x phase diagram for a system composed of Mg and Si. Made by faculty at the University of Colorado Boulder Departme...
Solid-liquid phase equilibrium and phase diagram of the ... The phase diagram belongs to a simple-type ternary system, and neither double salt nor solid solution was formed. Based on the phase diagrams of this system at 298.15 and 338.15 K, the cycle separation process for CsNO 3 recovery from the eluant of sodium nitrate mixture solution was evolved.

0 Response to "41 solid liquid phase diagram"
Post a Comment