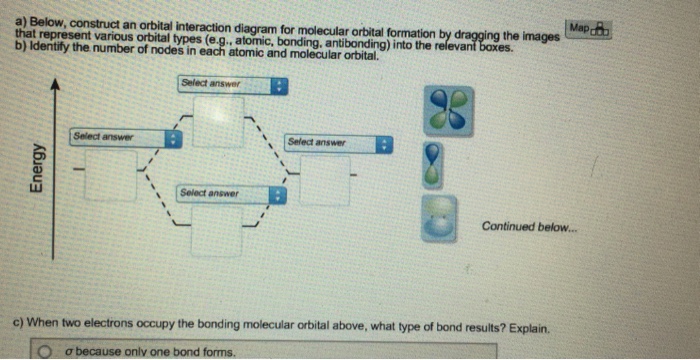
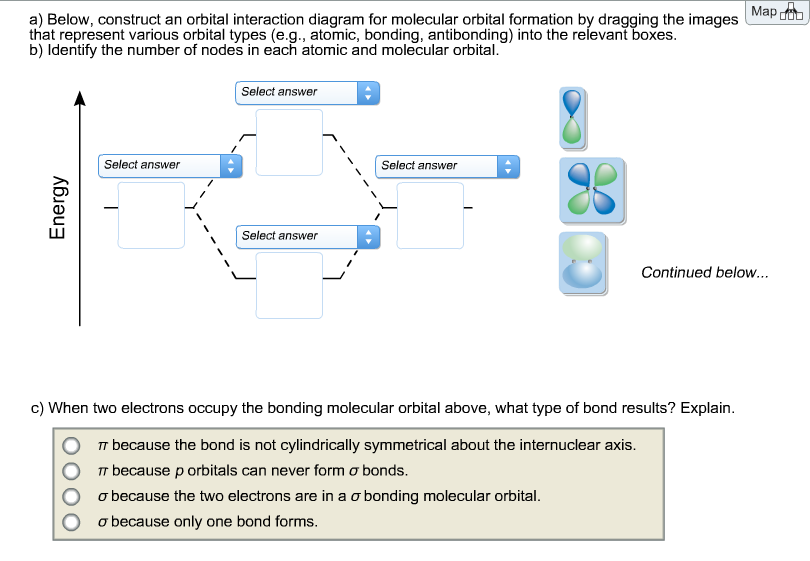
41 below construct an orbital interaction diagram
Step 3: Construct the orbital diagram for the ion. 82% (439 ratings) Procedure for Construct ing Molecular Orbital Diagram s B as ed on Hybrid Orbital s 1. Begin with the Lewis structure. 2. Decide how many orbital s each atom needs to make its sigma bonds and to hold its non-bonding electrons. A triple bond consists of one sigma and two pi bonds. Figure: A single bond consists of a single sigma bond, while a double bond requires both a sigma and a pi bond. Recall that atoms arrange electrons in orbitals, which include s and p orbitals. When molecules form, s and p orbitals from atoms combine to create hybrid orbitals.
The orbital diagram for the atom of cobalt is shown below. Mo diagram for hf the ao energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. Draw the orbital diagram for ion co 2.

Below construct an orbital interaction diagram
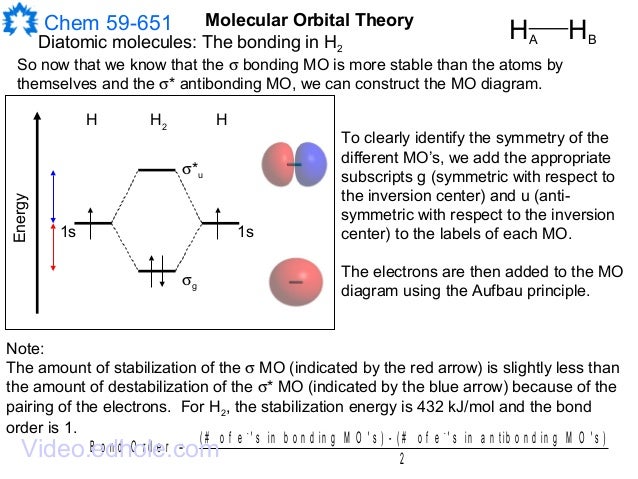
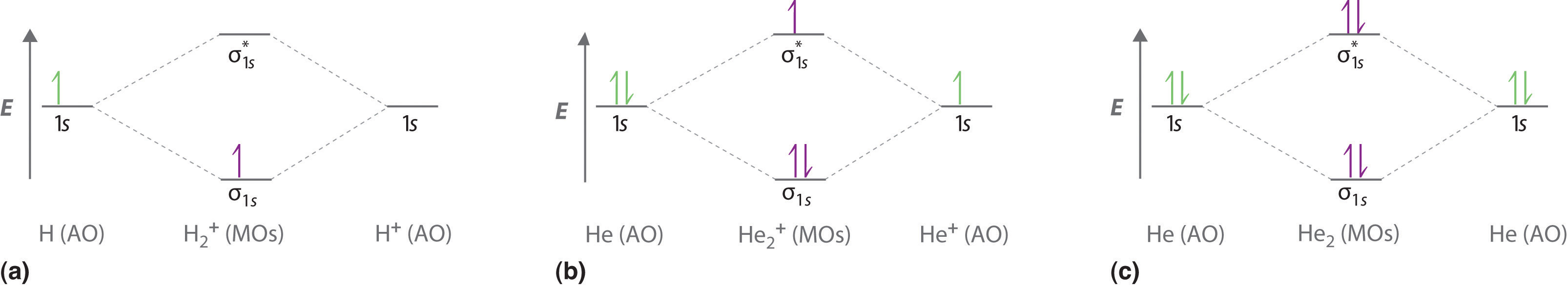
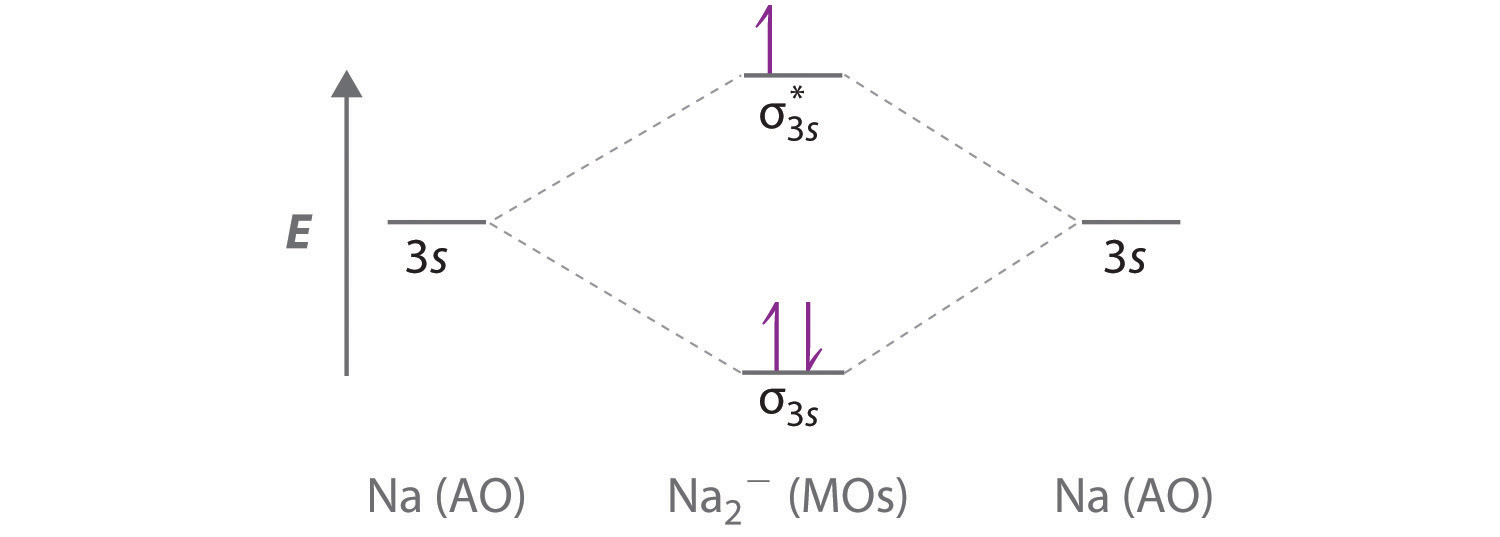
Energy-Level Diagrams. Because electrons in the σ 1 s orbital interact simultaneously with both nuclei, they have a lower energy than electrons that interact with only one nucleus. This means that the σ 1 s molecular orbital has a lower energy than either of the hydrogen 1s atomic orbitals. Conversely, electrons in the \( \sigma _{1s}^{\star } \) orbital interact with only one hydrogen ... Eye contact. To some, engaging in direct eye contact with strangers elicits a sense of dreadful anxiety. Many have a propensity for avoiding it altogether - whether they do so by staring at the ceiling, or by eyeing the irregularities between the coatings of paint on the walls, or, even, by observing the dips, dikes, and scars in the landscapes of their own palms - it is generally safe to say that people find many alternatives preferable to simply looking into another’s eyes, reserving the intim... Conclusion. SiO2 has a net dipole moment of zero. It has a linear electron and molecular geometry with a bond angle of 180 degrees and a hybridization of Sp. The Silicon dioxide Lewis structure has a total of 16 valence electrons. In the Lewis dot structure of SiO2, the formal charge is zero.
Below construct an orbital interaction diagram. SF4 Lewis Structure. Lewis structure is a pictorial representation of the bonds and valence electrons in the molecule. The bonds formed between two atoms are depicted using lines, whereas the valence electrons not forming any bonds are shown by dots. The valence electrons that participate in forming bonds are called bonding pairs of electrons ... Construct SALCs and the molecular orbital diagram for H\(_2\)O. This is the first example so far that is not a linear molecule. Water is a bent molecule, and so it is important to remeber that interactions of pendant ligands are dependent on their position in space. In the diagram below, the hydrogen atom interacts with one of the p orbitals on the oxygen, but it does not matter exactly which oxygen orbital we use. Figure \(\PageIndex{6}\): Molecular orbital interaction diagram for formation of hydroxide ion. In most cases, we could come up with the MO diagram in another way. As shown above, the Vanadium Electron Configuration of the element Vanadium is Ar 3d3 4s2. Therefore, now it is easier to understand the ground state, and the element Vanadium, its ground state is written as the following; [Ar] 3d 3 4s 2. How many must be thinking that what exactly is it, so to make it easier, the atomic number of the element ...
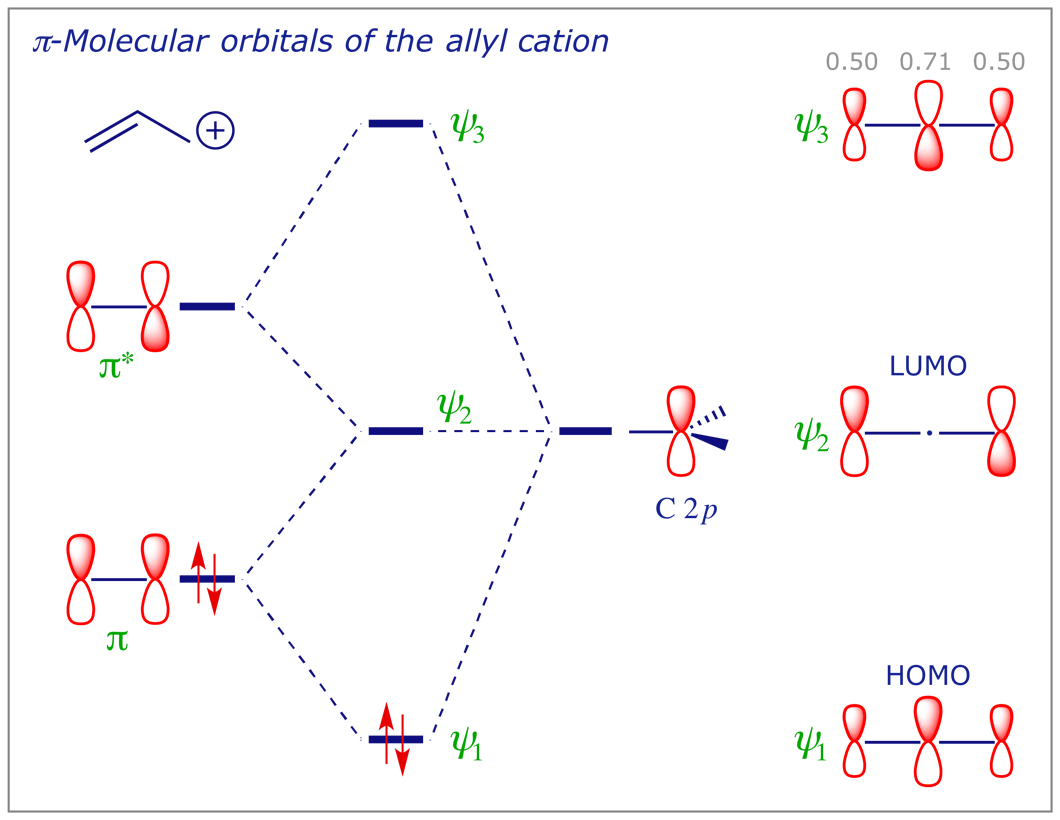
When I first heard about ATCOR, I didn't believe it. I figured it was a wildly unrealistic principle, and I couldn't find many resources about it to understand it. I thought this itself was very suspicious, since it was so "overpowered" if true, I would have expected people to have prepared detailed resources long ago to shout from the rooftops. However, I think I've finally just about managed to wrap my head around it for the most part, and now I feel like it could be surprisingly robust. I ... Eye contact. To some, engaging in direct eye contact with strangers elicits a sense of dreadful anxiety. Many have a propensity for avoiding it altogether - whether they do so by staring at the ceiling, or by eyeing the irregularities between the coatings of paint on the walls, or, even, by observing the dips, dikes, and scars in the landscapes of their own palms - it is generally safe to say that people find many alternatives preferable to simply looking into another’s eyes, reserving the intim... To make the analysis precise, one can construct the state correlation diagram for the general [4+2]-cycloaddition. As before, the ground state is the electronic state depicted in the molecular orbital correlation diagram to the right. This can be described as Ψ 1 2 π 2 Ψ 2 2, of total symmetry S 2 S 2 A 2 =S. [First](https://www.reddit.com/r/HFY/comments/ib293n/the_shoulders_of_orion_ch_1_first_contact/) [Previous](https://www.reddit.com/r/HFY/comments/m9fg9g/the_shoulders_of_orion_ch_6_claims/) Councilor Halon Va, former High Admiral of the combined Federation fleets, sat silently, observing the spectacle before him as his shuttle cruised towards the human ships. When news had first broken of the attack on Chelsith, human relief efforts had materialized at the edge of the system before even ...
To apply Molecular Orbital Theory to the diatomic homonuclear molecule from the elements in the second period. You are watching: Determine the bond order in a molecule or ion with 12 valence electrons. If we combine the splitting sjulianum.netes for the 2s and 2p orbitals, we can predict bond order in all of the diatomic molecules and ions composed of elements in the first complete row of the ... Two p-atomic orbitals (one from each nitrogen) atom combine to form two molecular orbitals, the bonding molecular orbital σ2px and antibonding molecular orbital σ*2px. The other four p-atomic orbitals (two from each nitrogen) atom combine to give four molecular orbitals, two bonding molecular orbitals i.e. π2py and π2pz, while two ... The remaining one is a non-bonding orbital but doubly field, which denotes the lone pair of phosphorus. Given below is the MO diagram of PF3 taking reference to which you can easily draw for PCl3. A MO diagram helps us to know about the bonding, bond order, bond angle, and bond length of any compound. Below is the video snippet attached for the ... Eye contact. To some, engaging in direct eye contact with strangers elicits a sense of dreadful anxiety. Many have a propensity for avoiding it altogether - whether they do so by staring at the ceiling, or by eyeing the irregularities between the coatings of paint on the walls, or, even, by observing the dips, dikes, and scars in the landscapes of their own palms - it is generally safe to say that people find many alternatives preferable to simply looking into another’s eyes, reserving the intim...
The effective coupling decreases then significantly to 26 meV (Table 2), showing that removing the nonorthogonality between the orbital sets of molecule A and B of the dimer decreases the orbital interactions between the two molecules. Hence, the overlap between the orbitals of the constituent molecules governs the interaction between the ...
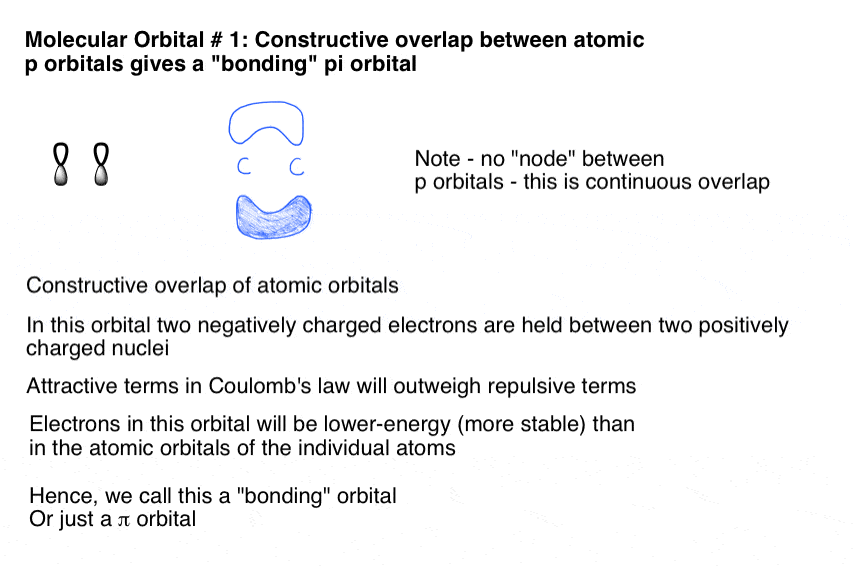
To showcase the relative energy levels of the AOs and the resultant MOs, we have the Molecular Orbital diagrams. The lower energy molecular orbital is the bonding and the higher energy is the anti-bonding orbital. The below-mentioned diagram gives us the individual MO diagrams of Oxygen and Fluorine separately, the atoms that make up a molecule ...
The d orbital splitting diagram is shown in a box. Suppose the diagram above is for a first row transition metal. The diagram for a second or third row metal is similar, but with stronger bonds. If the bonding interaction is stronger between the metal and ligand, then so is the antibonding interaction.
You can see that it has one s orbital and two p orbitals (the third p orbital is full), so it is designated as sp 2. When you draw an orbital picture of it, it has three total orbits, which are ...
Ground State Electron Configuration For Nitrogen. When we talk about the electronic configuration, then the ground state Nitrogen Electron Configuration is written as 1s 2 2s 2 2p 3.Below you can get the full image representation which will help you to understand the topic more easily.
About Priyanka. To read, write and know something new every day is the only way I see my day! Well, that rhymed. Hey folks, this is me, Priyanka, writer at Geometry of Molecules where I want to make Chemistry easy to learn and quick to understand.
Below are the orbitals that this compound occupies: One 3s orbital, Three 3p orbitals, One 3d orbital. You will be able to understand what we just told you better with the help of the above-given diagram. The hybridization of any compound depends solely and largely on its Steric number.
Considering inter-orbital and intra-orbital electron density-density interactions, we calculate superconducting phase diagrams. We find that the lattice distortions can induce unconventional ...
Here, Herbrych et al. predict that a large Coulomb interaction helps develop a spiral spin order, which is a topological state at the edge of a canonical superconductor with orbital degrees of ...
Molecular Orbital Theory and MO diagram of Dibromine (Br2) The MO diagram or Molecular Orbital diagram is an extension of the 3-dimensional molecular design and gives a better understanding of the structure of an atom. Molecular Diagram also reflects upon bond length, bond shape, bond energy, and the bond angle between 2 atoms.
This results in a triplet ground state. The finished valence molecular orbital diagram is pictured below. This problem has been solved! See the answer. The Molecular Organization diagram below is appropriate for B2. Based on this diagram, a)draw the molecular orbital filling diagram for B2. b) what is the bond order of B2. c) is B2 diamagnetic ...
Energy-Level Diagrams. Because electrons in the σ 1 s orbital interact simultaneously with both nuclei, they have a lower energy than electrons that interact with only one nucleus. This means that the σ 1 s molecular orbital has a lower energy than either of the hydrogen 1s atomic orbitals. Conversely, electrons in the \( \sigma _{1s}^{\star } \) orbital interact with only one hydrogen ...
The key to suspense is that it sets up a question, or several that the audience hopes to get an answer to, and delays that answer while maintaining their interest and keeping them guessing. The boy gives birth to demons, he can't help himself. That boy gets chased until he no longer runs, no longer walks, no longer stands, no longer thinks, and holds his breath for a few seconds. That boy sacrifices himself so that paradoxically the man can save the boy. Life rewards practical self-justifying o...
Answer: Hyperconjugation is the stabilising interaction that results from the interaction of the electrons in a σ-bond (usually C-H or C-C) with an adjacent empty or partially filled p-orbital or a π-orbital to give an extended molecular orbital that increases the stability of the system. In a...
Energy Bands. The energy spectrum result from solving the Equation (1) have large number of solutions, giving discrete energies E 1, k, E 2, k etc., for each value of k and is shown in Figure 4. Since these energies depend on k, they vary continuously as k is varied over its range of values.
(a) The schematic diagram of two-orbital TB model in a triangle lattice of orbitals locating, respectively, at top (front) and bottom (behind) surface of TI film. The phase diagrams of (b), (c) electronic states and (d)-(f) superconducting quasiparticles in the parameter space of Z t and Z b. The values of parameters used in the TB model are ...
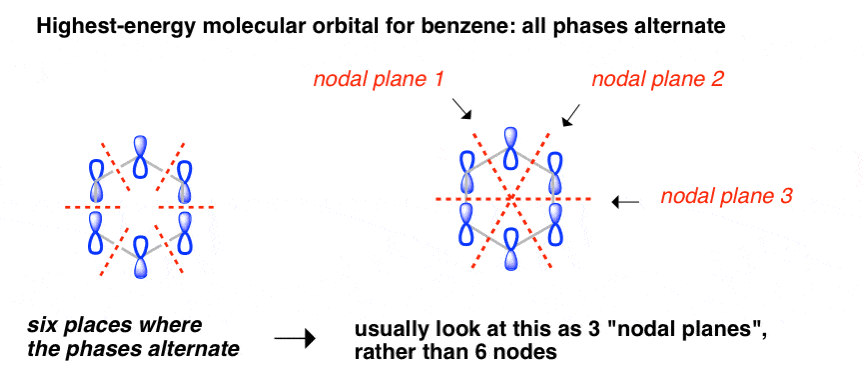
The diagram below shows the electron density distribution for the 1s, 2s and 3s orbitals with increasing distance from the nucleus ls 2s 3s ls 2s 3s Distance from the nucleus,r (a) The 3s orbital is said to have 2 'nodes'. Describe (or draw a diagram to show) where the nodes are found in the...
Conclusion. SiO2 has a net dipole moment of zero. It has a linear electron and molecular geometry with a bond angle of 180 degrees and a hybridization of Sp. The Silicon dioxide Lewis structure has a total of 16 valence electrons. In the Lewis dot structure of SiO2, the formal charge is zero.
Eye contact. To some, engaging in direct eye contact with strangers elicits a sense of dreadful anxiety. Many have a propensity for avoiding it altogether - whether they do so by staring at the ceiling, or by eyeing the irregularities between the coatings of paint on the walls, or, even, by observing the dips, dikes, and scars in the landscapes of their own palms - it is generally safe to say that people find many alternatives preferable to simply looking into another’s eyes, reserving the intim...
Energy-Level Diagrams. Because electrons in the σ 1 s orbital interact simultaneously with both nuclei, they have a lower energy than electrons that interact with only one nucleus. This means that the σ 1 s molecular orbital has a lower energy than either of the hydrogen 1s atomic orbitals. Conversely, electrons in the \( \sigma _{1s}^{\star } \) orbital interact with only one hydrogen ...

/chapter2/pages17and18/page17and18_files/moconnoncon.png)















0 Response to "41 below construct an orbital interaction diagram"
Post a Comment