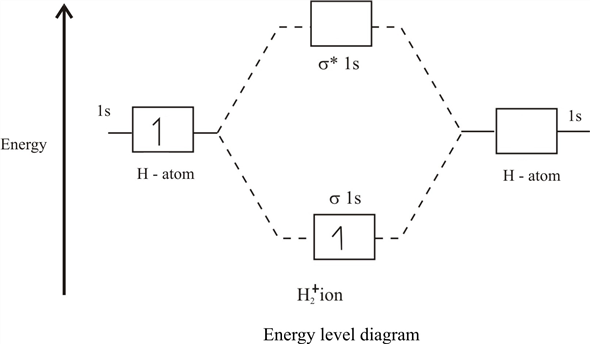
41 molecular orbital diagram for h2+
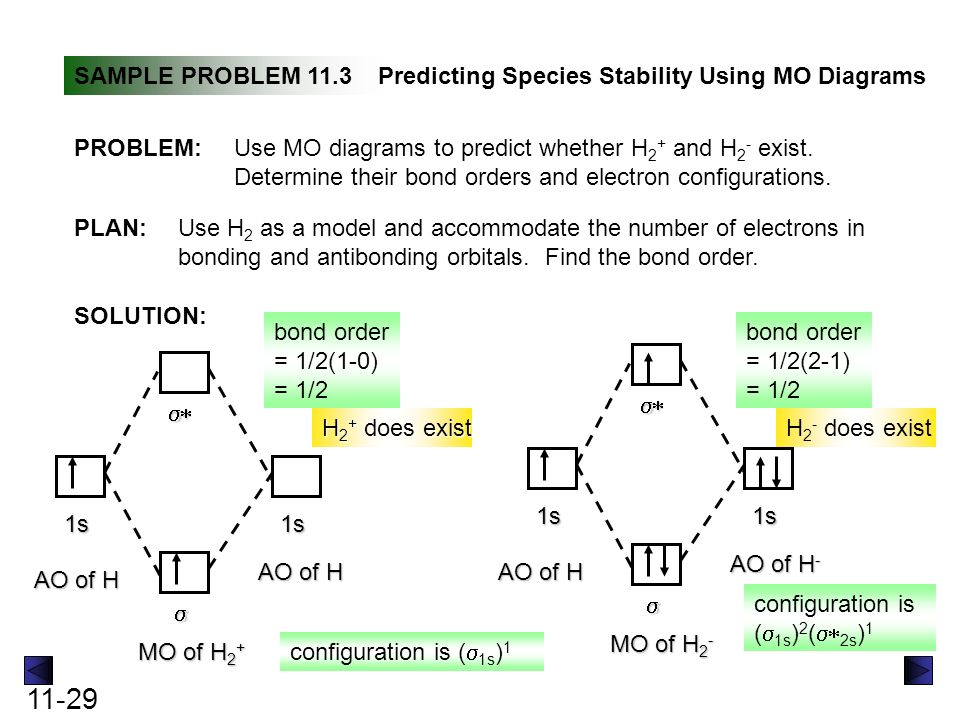
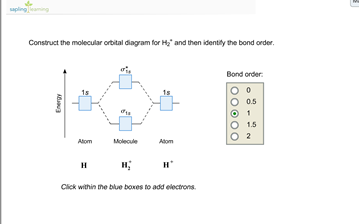
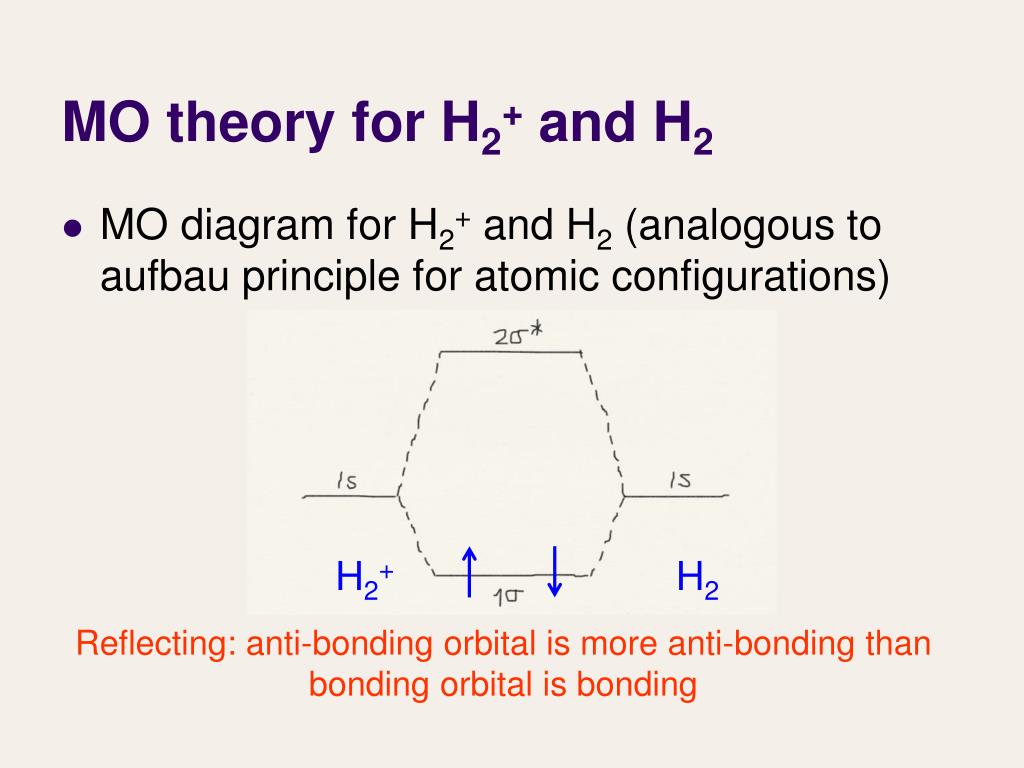
Problem: Construct the molecular orbital diagram for H2- and then identify the bond order. Click thin the blue boxes to add electrons.Bond order: a) 0 b) 0.5c) 1 d) 1.5e) 2 ... Construct the molecular orbital diagram for H2- and then identify the bond order. Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, "H"_2^(-) has three electrons while "H"_2^(+) has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one sigma_(1s) and one sigma_(1s)^"*" MO by conservation of orbitals.
August 5, 2021 - Interactive 3D chemistry animations of reaction mechanisms and 3D models of chemical structures for students studying University courses and advanced school chemistry hosted by University of Liverpool

Molecular orbital diagram for h2+
Description of the molecular orbitals of the h2 molecule with an introduction to molecular orbital diagrams. Each boron atom has one 2s and three 2p valence orbitals. In order to predict the bond order molecular orbital diagram for h2 is to be drawn. Molecular orbitals of h 2 the molecular orbital approach is one explanation for the ceh h bond. Answer (1 of 2): In order to predict the bond order, molecular orbital diagram for H2- is to be drawn. According to MOT number of atomic orbitals combined is equal to total number of molecular orbitals formed.Electronic configuration of H is 1s1. when two hydrogen atoms come closer, then on combi... Get access to the latest Bonding in Homonuclear Diatomic Molecules: H2, H2+, H2-, He2, He2+ prepared with IIT JEE course curated by Megha Khandelwal on Unacademy to prepare for the toughest competitive exam.
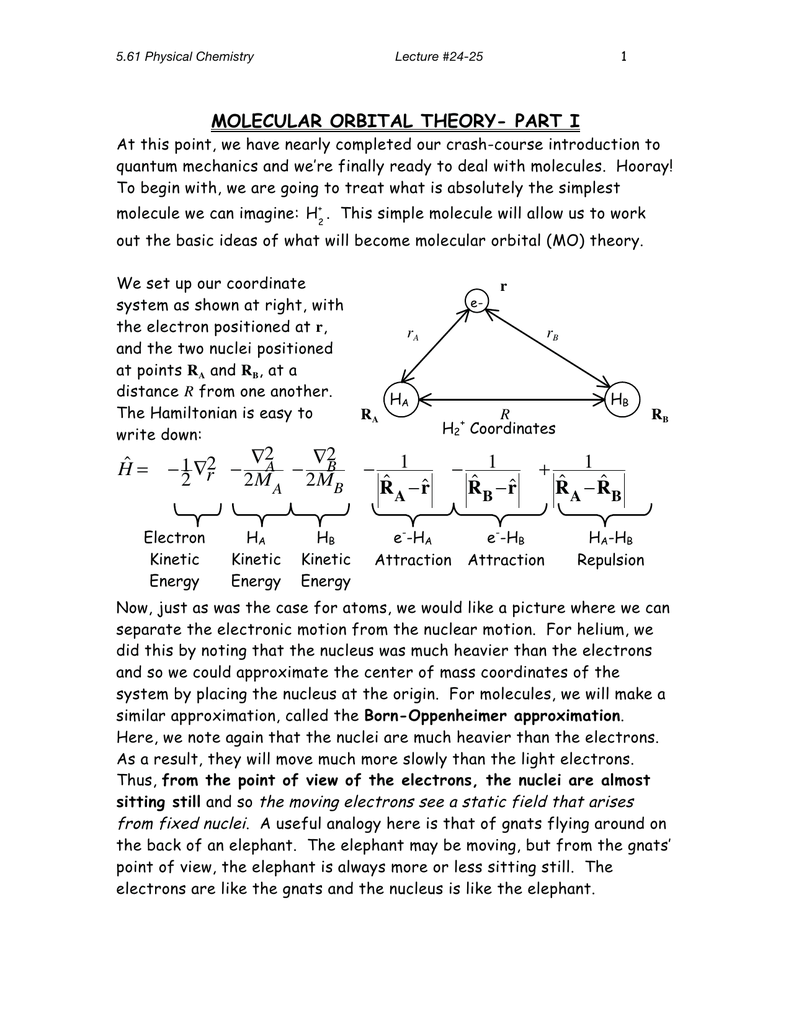
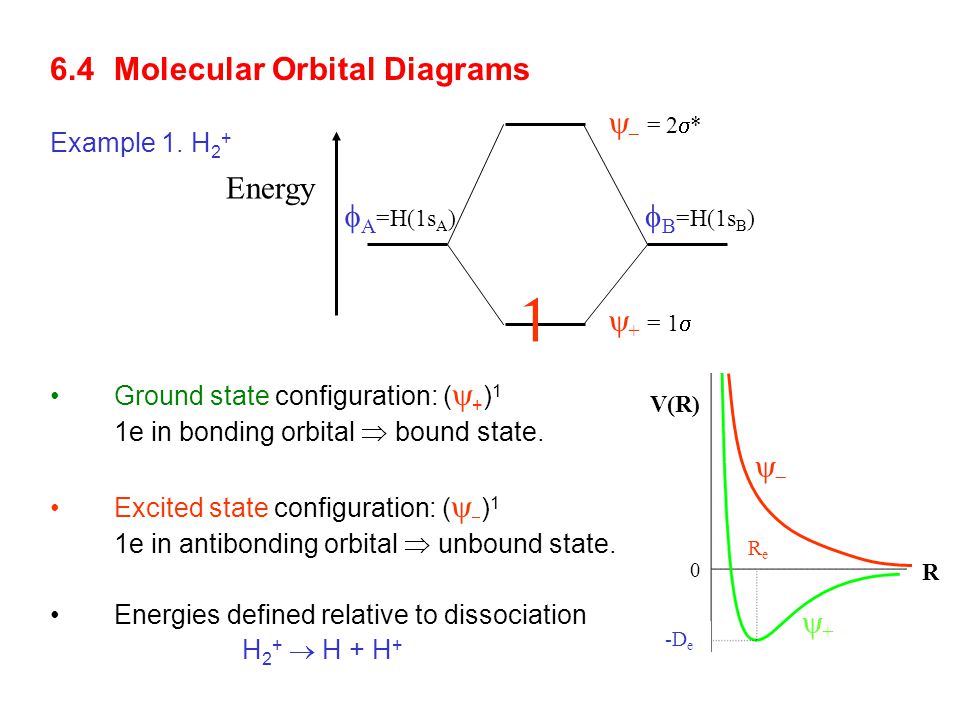
Molecular orbital diagram for h2+. Molecular Orbital (MO) Theory of the H2 molecule: Following the MO treatment of H2+, assume the (normalized) ground electronic state wavefunction is given by: gs ψψ αβ ... Qualitative MO theory orbital diagram for homonuclear diatomics composed of 1st or 2nd row elements: Principle 2 & 3: This interaction ... to the molecular orbitals, may also be represented in the form of an orbital (electron) energy diagram which shows the relative energies of the orbitals. In the specific case of hydrogen each of the isolated atoms has one electron in its 1s orbital and when the atoms combine to form H2 the two electrons ... Answer to Construct the molecular orbital diagram for H2+ and then identify the bond order.... Chemistry questions and answers. Construct the molecular orbital diagram for H2+ If all of the orbitals are unoccupied, place the corresponding token in the bin underneath the answer bank. os Answer Bank 11 1 1s 1s Energy 015 all of the orbitals are unoccupied Atom Molecule Atom HT h H Identify the bond order. 0 0.5 1 1.5 2.
Draw the molecular orbital energy level diagram of H2. Answer. Prev Question Next Question. Related Questions to study. The degenerate orbitals of ... T i 3 + (a q) is violet while T i 4 ... FREE Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click... December 19, 2016 - Discussed in this video are. C would this ion exist. Tikz Pgf Molecular Orbital Diagrams In Latex Tex Latex Stack ... May 18, 2018 - Brainly.in is a part of the largest social network for studying in a group. We provide the best tools for mutual help with school subjects. Join us!
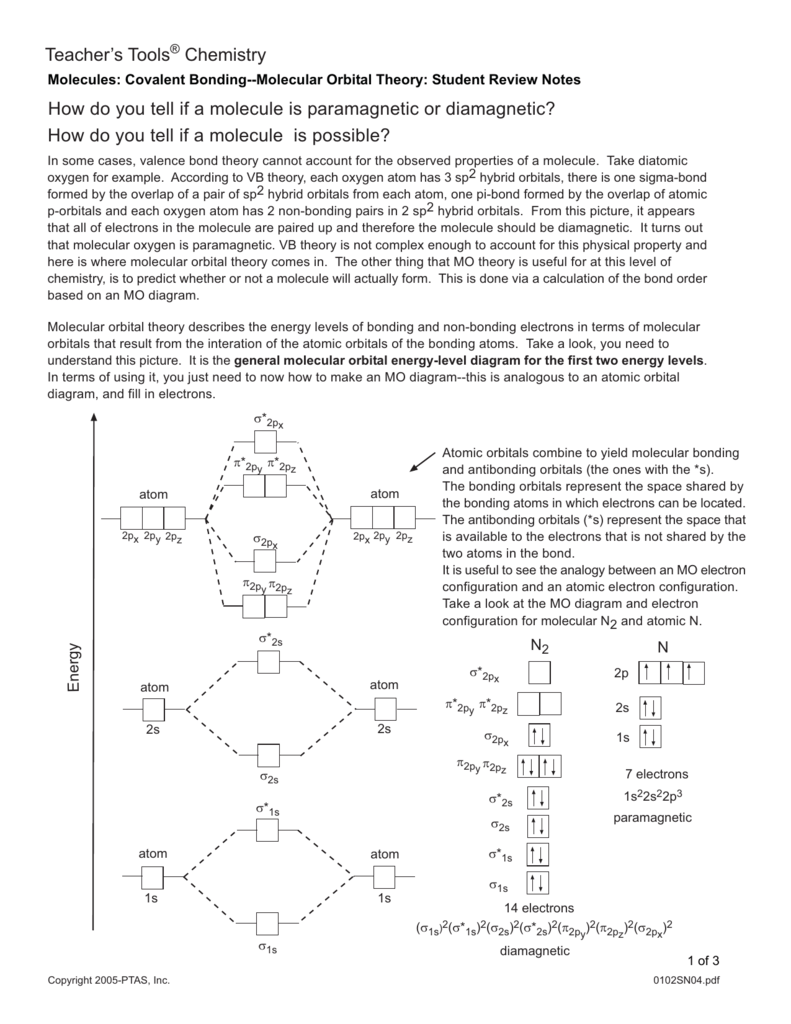
Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the energetically-favored configuration. Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ... 14+ H2 Molecular Orbital Diagram. A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. In molecular orbital theory bond order is defined as half of the difference between the number of bonding and antibonding electrons. The bond order of diatomic nitrogen is three and it is a diamagnetic molecule. Construct the molecular orbital diagram for h2 and then identify the bond order. According to mot number of atomic orbitals combined ...
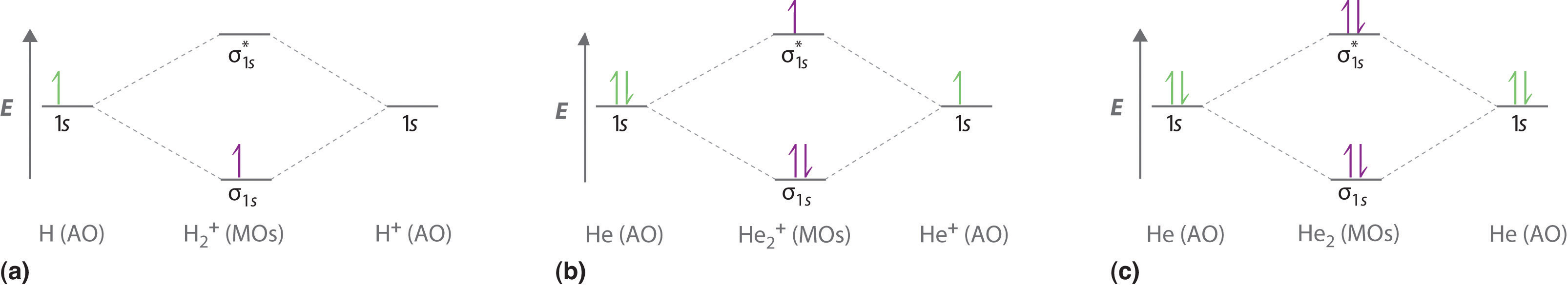
A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ...
The MO for $\ce{H2}$, which is shown in the figure below is taken from Wikipedia. The right side of the diagram you showed neither represents a hydrogen molecule, nor two independent (and hence equivalent) hydrogen atoms. ... How to explain the structure of HNO3 with a Molecular Orbital diagram? 7.

Use Molecular Orbital Theory To Determine Whether He2 2 Or He2 Is More Stable Draw The Molecular Orbital Diagram For Each And Explain Study Com
Construct the molecular orbital diagram for h2 and then identify the bond order. In fact they do. πε and jr k r mr lr defined explicitly in atkins. The orbital correlation diagram in predicts the same thing two electrons fill a single bonding molecular orbital. The procedure can be introduced by considering the h2 molecule.
8:50Hello dear students. In this video we will study how to calculate and what is the bond order of H2 , H2+ and H2 ...1 Jul 2021 · Uploaded by My chemistry teacher
This video discusses how to draw the molecular orbital (MO) diagram for the H2+ ion. The bond order of H2+ is also calculated and the meaning of this number ...
1)H2+. Molecular orbital energy level for H2+. The electronic configuration of H2+. Answer to Create an MO diagram for H2+ H2 and H Post the Lumo, lumo -, homo, homo + near its energy level. Molecular Orbital (MO) Theory of the H2 molecule: Following the MO treatment of H2+, assume the (normalized) ground electronic state wavefunction is .
December 31, 2016 - Construct the molecular orbital diagram for h2 and then identify the bond. Mykale 26 november 2018 0037. Introduction to...
Answer and Explanation: 1. This question deals with the molecular orbital (MO) diagram of the simple diatomic hydrogen molecule. VSEPR theory describes this as two hydrogen atoms forming a single ...
A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. According to the molecular orbital theory, in a supposed He2 molecule, both the if we draw its MOT DIAGRAM, 2 e's enter the Bonding molecular Orbital and 2 .
March 2, 2021 - https://chem.libretexts.org/@a...A_molecules/2.3%3A_Homonuclear_Diatomic_Molecules_-_Molecular_Orbital_(MO)_Theory/2.3b%3A_MO_theory_of_bonding_in_H ... Note that there is a nodal plane in the anti-bonding MO. ... For H2, bond order = 1/2 (2-0) = 1, which means H2has only ...
August 15, 2020 - Check out our new LibreCommons search portal · Describe the hydrogen molecule in light of the following:
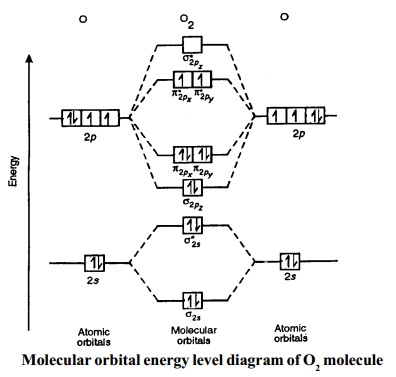
Molecular orbital diagram of h2. When creating the molecular orbitals from the p orbitals notice the three atomic orbitals split into three molecular orbitals a singly degenerate σ and a doubly degenerate π orbital. Molecular orbitals of h2 and he2. Bonding mos antibonding mos and bond order. Evaluate the ground state electronic energy based ...
LCAO MO Energy Diagram for H2 Energy H-H ∆E1 ∆E2 • ∆E2> ∆E1, so the antibonding orbital is always more anti-bonding than the bonding orbital is bonding H2molecule: two 1s atomic orbitals combine to make one bonding and one antibonding molecular orbital. Ha Hb
Construct the molecular orbital diagram for h2 and then identify the bond order. Consult a diagram of electron orbital shells. A triple covalent bond three. Molecular orbital mo theory of the h2 molecule. In molecular orbital theory bond order is defined as half of the difference between the number of bonding and antibonding electrons.
Molecular orbitals of h2 and he2. The molecular orbital energy level diagram for the h2 ion. Suppose that the ion is excited by light so that an electron moves from a lower energy to a higher energy molecular orbital. πε and jr k r mr lr defined explicitly in atkins. Sketch the molecular orbitals of the h2 ion and draw its energy level diagram.
November 23, 2015 - 12/20: Andy's paper on supported nanocrystal catalysts is published in Chem. Mater.! 10/20: Our joint paper with the Klimov group using ALD to produce CMOS circuit elements from CIS quantum dot films is published in Nature Comms. 9/20: Our paper with Adam Moule's group on electron tomography ...
5:31and anti bonding molecular orbitals are empty. So as all the electrons in hydrogen molecule are paired up, it ...8 Jun 2020 · Uploaded by Edmerls
We Say That If We Bring Two Hydrogen Atoms Closer To Each Other Their Wavefunctions Can Interfere To Form A Bonding And An Antibonding Mo What Happens In The Case When We
Science. Chemistry. Chemistry questions and answers. Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes to add electrons. Question: Construct the molecular orbital diagram for H2- and then identify the bond order.
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
7:24"molecular orbital diagram hydrogen molecule ion [H2+]"in molecular orbital theory. Watch later. Share ...16 Jan 2020 · Uploaded by Personal Tutor
eigenvectors are the coefficients of the molecular orbitals, written as sums of AOs: N ψα( ) = ∑c i αφ i r AO (r) i=1 In general, we will obtain N molecular orbitals out of N atomic orbitals. This step is precisely the same as what we did for H2+, just generalized to the Norbital case. We note that for anything larger than a 2by2, it
In this video, we take a detailed look at the molecular orbitals of the H2 molecule, with an introduction to molecular orbital diagrams. Discussed in this v...

Artificial Nodes In The H2 Wave Functions Expanded Using Gaussian Type Orbitals Or Laguerre Type Orbitals Sciencedirect
FREE Answer to Construct the molecular orbital diagram for H2+ and then identify the bond order.1 answer · 1 vote: Molecular orbitals: Molecular orbitals are formed by linear combination of atomic orbitals. Atomic orbitals and molecular orbitals of a molecule can be ...
March 10, 2016 - Molecular orbital energy level diagrams of certain diatomic Homo nuclear molecules and molecular ions · The filling of molecular orbitals is governed by the following principles. ... (iii)Hund's rule of maximum multiplicity. Now, let us consider some examples of homo nuclear diatomic molecules. 1. Hydrogen molecule, H2...
Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here. Chemical bonding - Molecular orbitals of H2 and He2: The procedure can ...

Construct The Molecular Orbital Diagram For N2 And Then Identify The Bond Order What Is The Homeworklib
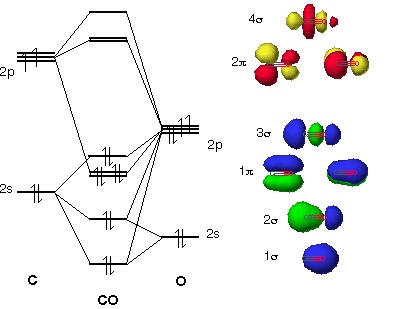
Oxygen has a similar setup to H2, but now we consider 2s and 2p orbitals. When creating the molecular orbitals from the p orbitals, notice the three atomic orbitals split into three molecular orbitals, a singly degenerate σ and a doubly degenerate π orbital.
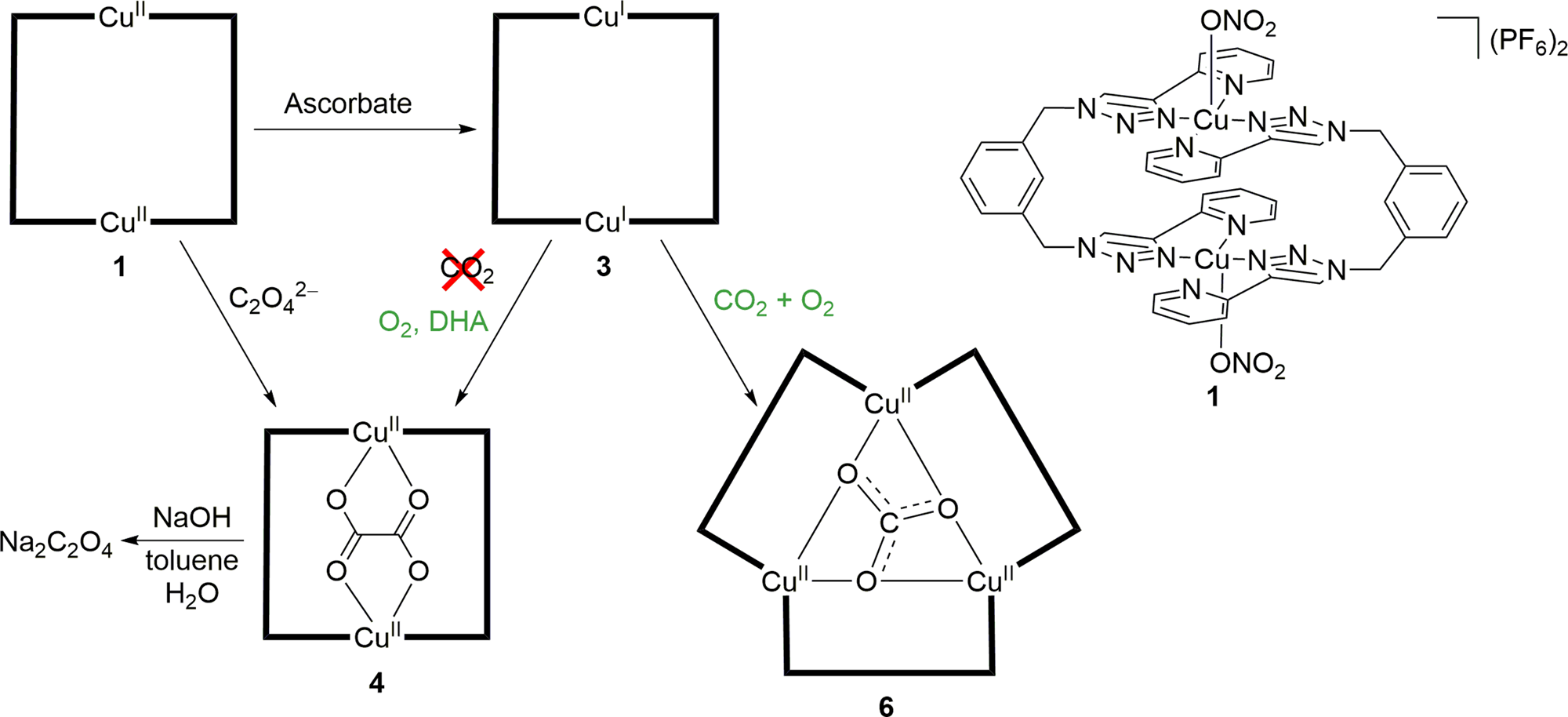
Oxalate Production Via Oxidation Of Ascorbate Rather Than Reduction Of Carbon Dioxide Nature Communications
The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H 2 molecule is shown in Figure 13. On either side of the central ladder are shown the energies of the 1 s orbitals of atoms A and B, and the central two-rung ladder shows the energies of the bonding and antibonding ...
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
Energy level diagram for Molecular orbitals. ... If N b = Na,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of electron in the bonding molecular orbitals. 2) Stability of molecules in terms of bond order.
It is common to omit the core electrons from molecular orbital diagrams and configurations and include only the valence electrons. Figure 8. This is the molecular orbital diagram for the homonuclear diatomic Be 2 +, showing the molecular orbitals of the valence shell only. The molecular orbitals are filled in the same manner as atomic orbitals ...
Molecular Orbital Diagram for Hydrogen Gas (H2).Fill from the bottom up, with 2 electrons total.Bonding Order is 1, and it is Diamagnetic.sigma2s(2)Check me ...
D.Construct the molecular orbital diagram for H2^+ and then identify the bond order. *need before 7:00; Question: A.Construct the molecular orbital diagram for H2^2+ and then identify the bond order. B.Construct the molecular orbital diagram for H2 and then identify the bond order.
3:31For the ion H2+: a) Draw the molecular orbital diagram. b) Calculate the bond order. c) Would this ion exist?2 Nov 2012 · Uploaded by Professor Heath's Chemistry Channel
Professor Patricia Shapley, University of Illinois, 2011
Get access to the latest Bonding in Homonuclear Diatomic Molecules: H2, H2+, H2-, He2, He2+ prepared with IIT JEE course curated by Megha Khandelwal on Unacademy to prepare for the toughest competitive exam.
Answer (1 of 2): In order to predict the bond order, molecular orbital diagram for H2- is to be drawn. According to MOT number of atomic orbitals combined is equal to total number of molecular orbitals formed.Electronic configuration of H is 1s1. when two hydrogen atoms come closer, then on combi...
Description of the molecular orbitals of the h2 molecule with an introduction to molecular orbital diagrams. Each boron atom has one 2s and three 2p valence orbitals. In order to predict the bond order molecular orbital diagram for h2 is to be drawn. Molecular orbitals of h 2 the molecular orbital approach is one explanation for the ceh h bond.












0 Response to "41 molecular orbital diagram for h2+"
Post a Comment