41 examine the following phase diagram and determine what phase(s) exists at point a.
Examine the following phase diagram and determine what phase exists at point F. A. vapor + liquid B. vapor C. liquid D. solid E. supercritical fluid. B. vapor. Examine the following phase diagram and determine what phase exists at point F Vapor When the electron cloud of a molecule is easily distorted, the molecule has a high _____________.
A typical phase diagram for a pure substance is shown in Figure 1. ... At pressures lower than the triple point, water cannot exist as a liquid, ...
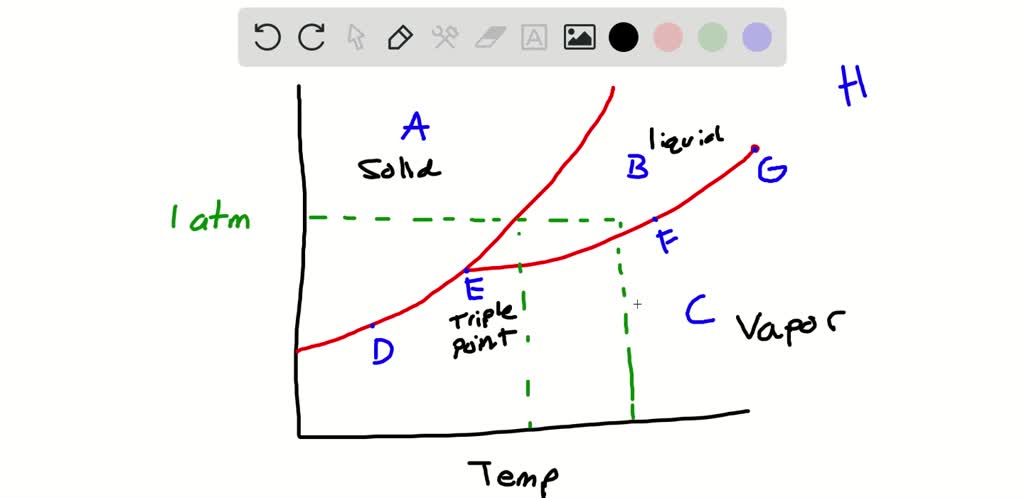
Examine the following phase diagram and determine what phase(s) exists at point a.
Examine the following phase diagram and identify the feature represented by point A. A. melting point B. critical point C. triple point D. sublimation point E. boiling point 2. Examine the following phase diagram and determine what phase exists at point F. A. vapor + liquid B. vapor C. liquid D. solid E. supercritical fluid 1 Using the phase diagram for water, we can determine that the state of water at each temperature and pressure given are as follows: (a) solid; (b) liquid; (c) liquid; (d) gas; (e) solid; (f) gas. Check Your Learning What phase changes can water undergo as the temperature changes if the pressure is held at 0.3 kPa? If the pressure is held at 50 kPa? 11 Oct 2012 — Examine the following phase diagram and determine what phase exists at point F. SEE QUESTION 14 Image A) vapor + liquid. B) vapor. C) liquid
Examine the following phase diagram and determine what phase(s) exists at point a.. Transcribed image text: 1 pe QUESTION 19 Examine the following phase diagram and determine what phase exists at point F. Teeper INote the phase diagram does ... Question: examine yhe following phase diagram and determine what phase exists at point F. This problem has been solved! See the answer ...Missing: s) | Must include: s) Problem: Examine the following phase diagram and identify the feature represented ... Critical Point: the point at which the liquid and gas phases coexist.1 answer · Top answer: Critical point A typical phase diagram for a pure substance is shown in Figure 1. ... At pressures lower than the triple point, water cannot exist as a liquid, ...
... the next question prevents changes to this answer, Question 2 Examine the following phase diagram and determine what phase exists at point F. Pressure . Examine the following phase diagram and determine what phase exists at point C. 1. gas and liquid. 2. gas. 3. liquid. 4. solid. 5. supercritical fluid. Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash Chemistry questions and answers. Examine the following phase diagram and determine what phase exists at point 760 som Temperature A) supercritical fluid B) liquid C) vapor+liquid D) vapor E) solid The phase diagram of a substance i s given below. This substance is a at 25°C and 1.0 atm. 1.5 P (atm) 1.0 0.5T -10 0 10 20 30 40 50 60 70 T ('C) A) gas B) crystal C) supercritical fluid D) solid.
11 Oct 2012 — Examine the following phase diagram and determine what phase exists at point F. SEE QUESTION 14 Image A) vapor + liquid. B) vapor. C) liquid Using the phase diagram for water, we can determine that the state of water at each temperature and pressure given are as follows: (a) solid; (b) liquid; (c) liquid; (d) gas; (e) solid; (f) gas. Check Your Learning What phase changes can water undergo as the temperature changes if the pressure is held at 0.3 kPa? If the pressure is held at 50 kPa? Examine the following phase diagram and identify the feature represented by point A. A. melting point B. critical point C. triple point D. sublimation point E. boiling point 2. Examine the following phase diagram and determine what phase exists at point F. A. vapor + liquid B. vapor C. liquid D. solid E. supercritical fluid 1






0 Response to "41 examine the following phase diagram and determine what phase(s) exists at point a."
Post a Comment