39 nitrogen molecular orbital diagram
A blank molecular orbital diagram (Figure 2) has been provided to help you. Physics of materials. Gaseous nitrogen has a density of 1.17 kg/m3 and liquid nitrogen has a density of 810 kg/m3. [The relative molecular mass of nitrogen is 28.0] What is the mean volume per nitrogen molecule in each case? What is the mean separation See also: Molecular orbital theory and Molecular orbital diagram ... molecular orbitals formed must be equal to the number of atomic orbitals in the ...
May 17, 2019 - Answer (1 of 2): Let me explain the molecular orbital diagram of N2 using its diagram. one atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons so first 2 electrons go in 1s sigma bond next 2 in 1s sigma anti bond orbital next 2 in 2s sigma bond orbital next 2 in 2s sigma...
Nitrogen molecular orbital diagram
I'm trying to understand how to draw molecular orbital diagrams, since I'd like to use the molecular orbitals to determine the total state of the ... March 20, 2019 - Energy level diagram for Molecular orbitals The first ten molecular orbitals may be arranged in order of energy as follow: σ(1s) Compass · Tables · Index · Introduction · Professor Patricia Shapley, University of Illinois, 2012
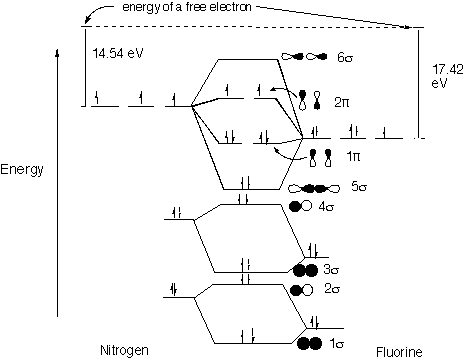
Nitrogen molecular orbital diagram. The findings regarding the node planes of the lowest unoccupied molecular orbitals (LUMOs) of C 70 and both the highest occupied molecular orbitals (HOMOs) and the LUMO of the C 70 anion suggest that electron tunneling of pristine C 70 prolate spheroidal fullerene could be better in the major axis orientation when facing the gate electrode than ... September 21, 2015 - I have been taught that the MO diagram is different for molecules with 14 or less electrons than the one used for molecules with 15 or more electrons. For $\ce{N2}$ the orbitals in increasing ene... Quantum-chemical calculation of most important parameters of molecular and electronic structures of octa-carbon C8 having cubic form (bond lengths, bond and torsion angles) using CCSD(T)/QZVP and DFT B3PW91/QZVP methods, has been carried out. NBO analysis data and HOMO/LUMO images for this compound are presented, too. Good agreement was found between the structural data obtained using the ... October 15, 2019 - The molecular orbital energy level diagram of N2 is given in fig. The bond order of N2 can be calculated as follows: Here, Nb=8 and Nb=2 Bond order =2Nb−Na=28−2=3 · Nature of bond: A bond order of 3 means that a triple bond is present in a molecule of nitrogen.
Presented diagram shows how the atomic orbitals of two N atoms combine to form molecular orbitals of nitrogen molecule: each nitrogen atom has three one-occupued p-orbitals and when the atoms interact they produce three bonding molecular orbitals of nitrigen molecule - one of sigma-type(symmetry) ... So, it will have some net magnetic moment. Atomic number of nitrogen is seven. Therefore in ${N_2}$ there are a total fourteen electrons. But $N_2^ - $ has one electron more than that of nitrogen molecule. So, total electrons are fifteen. Molecular orbital diagram of $N_2^ - $ is shown below: The nitrogen atom is less electronegative than the oxygen atoms so its atomic orbitals are a little higher in energy. By Hund's rule, the electron configuration of carbon, which is 1s 2 2s 2 2p 2, is understood to correspond to the orbital diagram shown in c. Experimentally, it is found that the ground state of a neutral carbon atom does indeed contain two unpaired electrons. Draw an orbital diagram for nitrogen, Z = 7. ১০ ফেব, ২০২১
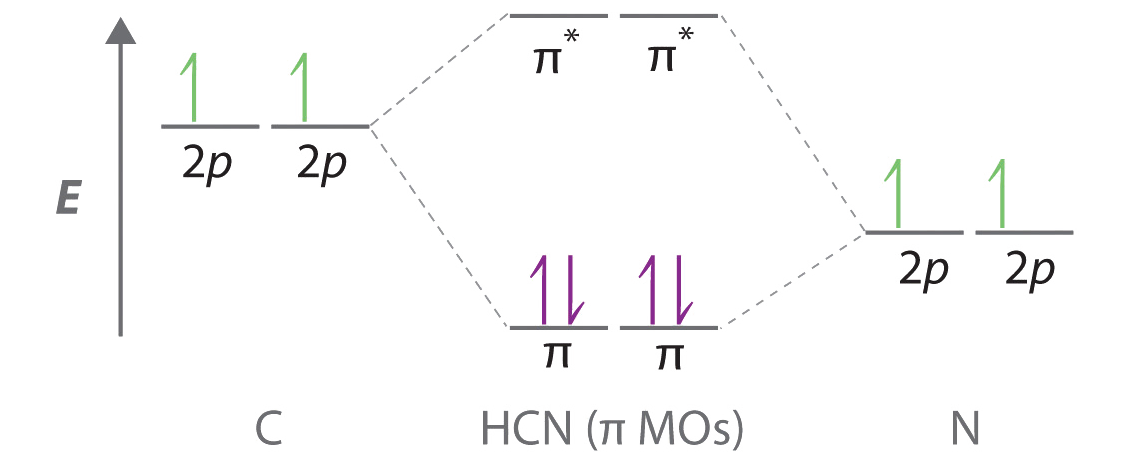
The MO method for N2+ gives the bond order equal to 2.5. But first, we look at the diagram of molecular orbitals for N2 (the bond order for the nitrogen molecule is 3). the N2+ molecule). That is, the bond order for N2+ is 2.5. In the HCN molecule, C atom includes sp - hybridized orbital, since it will combine with only two other atoms to form HCN. One of the sp - hybrid orbitals of Carbon atom overlaps with 1 s orbital of H atom, while other sp - hybrid orabital mixes with one of Nitrogen's atom's three atomic p orbitals which were unhybridized. A guide for alien Species concerning Humans by a Human ​ Written in Union Standard Machine Communication Language (USMCL) by Henry Katou Vizex Machine Translator recommended for reading ​ Section 1: Common misconceptions regarding Hummans ​ I have met individuals of a grand total of 3 non-Human Species and have already discovered a plethora of astonishing misconceptions about my Species. I am a humble molecular network engineer, but I would like to offer t... How to draw the molecular orbital energy level diagram for Nitrogen N2
The energy of , molecular orbital is greater than and molecular orbitals in nitrogen molecule. Write the complete sequence of energy levels in the increasing order of energy in the molecule. Compare the relative stability and the magnetic behaviour of the following species. <br> 24554767 .
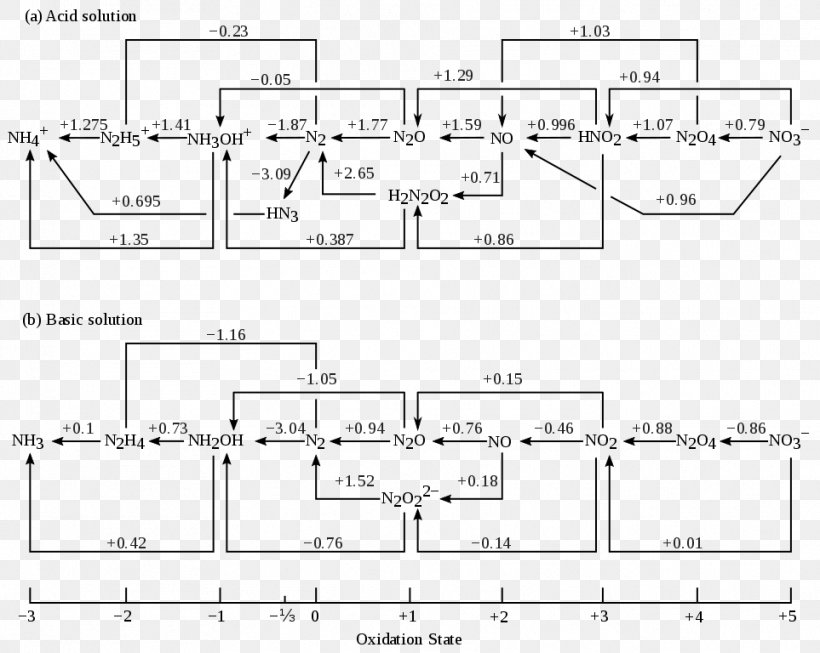
Frost Diagram Nitrogen Molecular Orbital Diagram Wiring Diagram Png 963x768px Watercolor Cartoon Flower Frame Heart Download
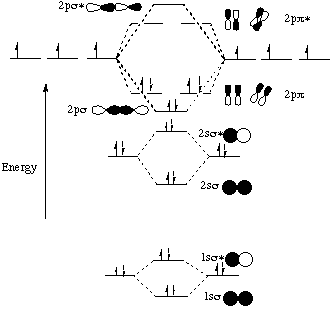
July 8, 2019 - Nitrogen molecule (N3) - N (7) 1s2 2s2 2p3 When we construct the MO diagram for nitrogen , we will only draw the valence 2s and 2p orbitals. In nitrogen MO , there is a slight change in MO diagram when compared to the MO diagrams of O2 , F2 molecules.Here,the σ2p orbital is higher in energy ...
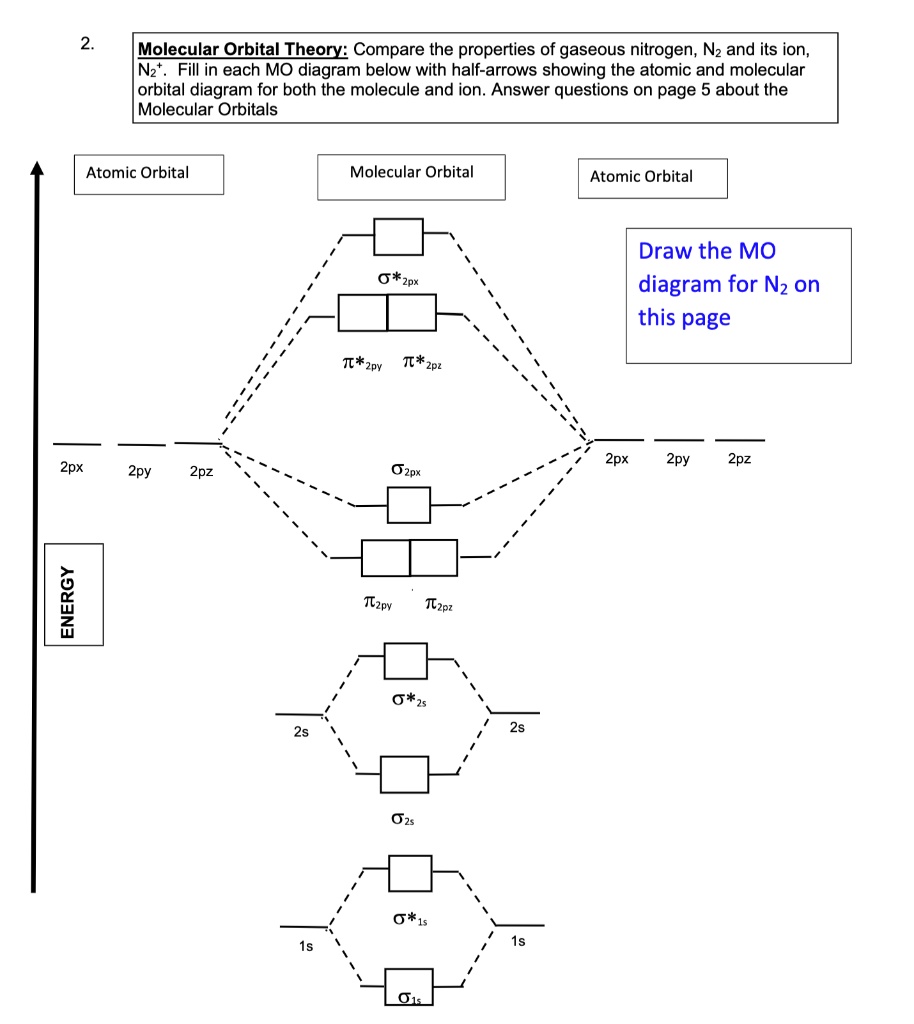
Solved Molecular Qrbital Theory Compare The Properties Of Gaseous Nitrogen Nz And Its Ion Nz Fill In Each Mo Diagram Below With Half Arrows Showing The Atomic And Molecular Orbital Diagram For Both The
May 18, 2019 - Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. The p orbitals combine to produce a sigma and two perpendicular pi bonds. Three filled bonding orbitals… ... Hydrogen | Fluorine | Nitrogen | Hydrogen Fluoride | Carbon Monoxide | Methane | Ammonia ...
8 - Drawing Molecular Orbital Diagrams. Abstract (TL;DR) Molecular orbital diagrams are a fantastic way of visualizing how molecular orbitals form using what we already understand about sigma and pi bonds. Depending on if it is a homonuclear case, where the bonding atoms are the same, or a heteronuclear case, where the bonding atoms are ...
14+ N2 Mo Diagram. With mo diagrams, we can predict the number of bonds in diatomic molecules. Molecular orbital diagram for nitrogen gas (n2) use aufbau and hund to fill with 10 valence electrons you get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2). N2 2 Molecular Orbital Diagram — UNTPIKAPPS from www.untpikapps.com Thus if we know…
January 4, 2021 - Because NO has an odd number of valence electrons (5 from nitrogen and 6 from oxygen, for a total of 11), its bonding and properties cannot be successfully explained by either the Lewis electron-pair approach or valence bond theory. The molecular orbital energy-level diagram for NO (Figure ...
A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) method in particular.
Molecular Orbital theory is a concept of quantum mechanics that attempts to explain the chemical bonding inside any molecule. In this theory, we get to know that valence electrons can be shared amongst all constituent atoms and atomic orbitals from different atoms combine to form molecular orbitals ( MOs ).
The unhybridized p atomic orbitals are used to form π bonds. Two unhybridized p atomic orbitals each from a different atom overlap side to side, resulting in a shared electron pair occupying the space above and below the line joining the atoms (the internuclear axis).. What is an unhybridized 2p orbital?, The unhybridized 2p orbital is directed perpendicular to the plane that contains the ...
Sigma Bonding and Antibonding Orbitals ... Pi Bonding and Antibonding Orbitals ... Molecular Orbital Diagram (N 2 )
In this video we will draw the molecular orbital diagrams for diatomic nitrogen, carbon and boron. We will also calculate their bond order and determine if t...
Molecular interfaces formed between metals and molecular compounds have enormous potential as building blocks for future opto-electronics and spin-electronics devices. Transition metal ...
NH3 Lewis Structure, Molecular Geometry, Hybridization, Bond Angle & Shape. Ammonia is a colorless compound, used in making fertilizers. It is a stable hydride formed of one nitrogen and three hydrogen atoms. The molecule has a pungent smell. It can form an NH4+ ion by accepting a proton.
... orbitals: s, p, d Shapes => Molecular Orbital Theory: Energy level diagram for molecular orbitals => Electronic configuration of a molecule and its ...

Frost Diagram Nitrogen Molecular Orbital Diagram Wiring Diagram Nitrogen Angle Text Rectangle Png Pngwing
A nitrogen atom has seven electrons. In the ground state, they are arranged in the electron configuration 1s 2 2s 2 2p 1 x 2p 1 y 2p 1 z.It therefore has five valence electrons in the 2s and 2p orbitals, three of which (the p-electrons) are unpaired. It has one of the highest electronegativities among the elements (3.04 on the Pauling scale), exceeded only by chlorine (3.16), oxygen (3.44 ...

Scielo Brasil A Brief Introduction To Molecular Orbital Theory Of Simple Polyatomic Molecules For Undergraduate Chemistry Students A Brief Introduction To Molecular Orbital Theory Of Simple Polyatomic Molecules For Undergraduate
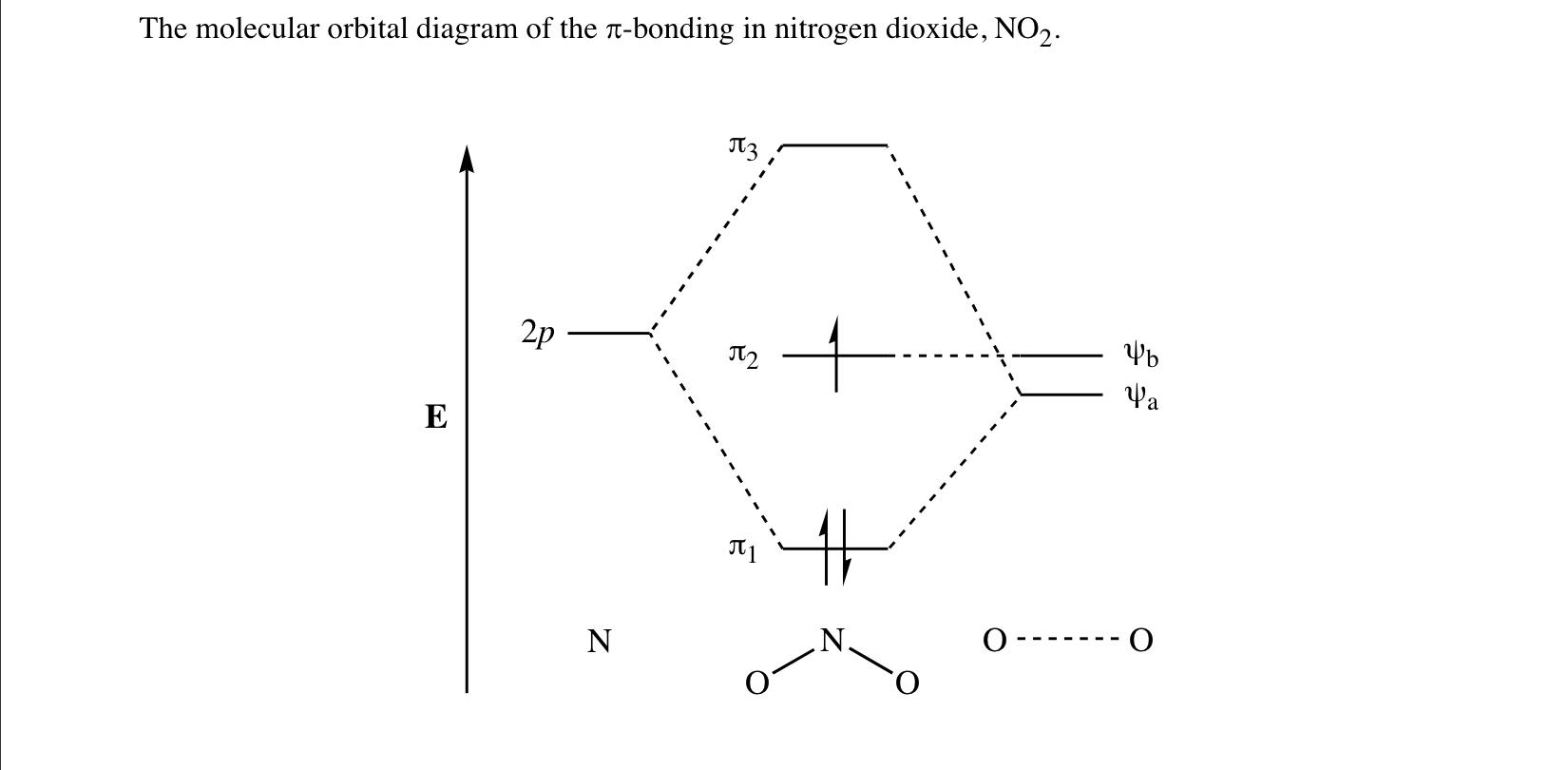
A molecular orbital (MO) diagram explains the chemical bonding in molecules by energy level diagrams. They were proposed by Robert S. Mulliken and Friedrich Hund in 1928. As we know N2O4 molecule is a dimer of the NO2 molecule, hence we'll discuss molecular orbital diagram of NO2 molecule first.
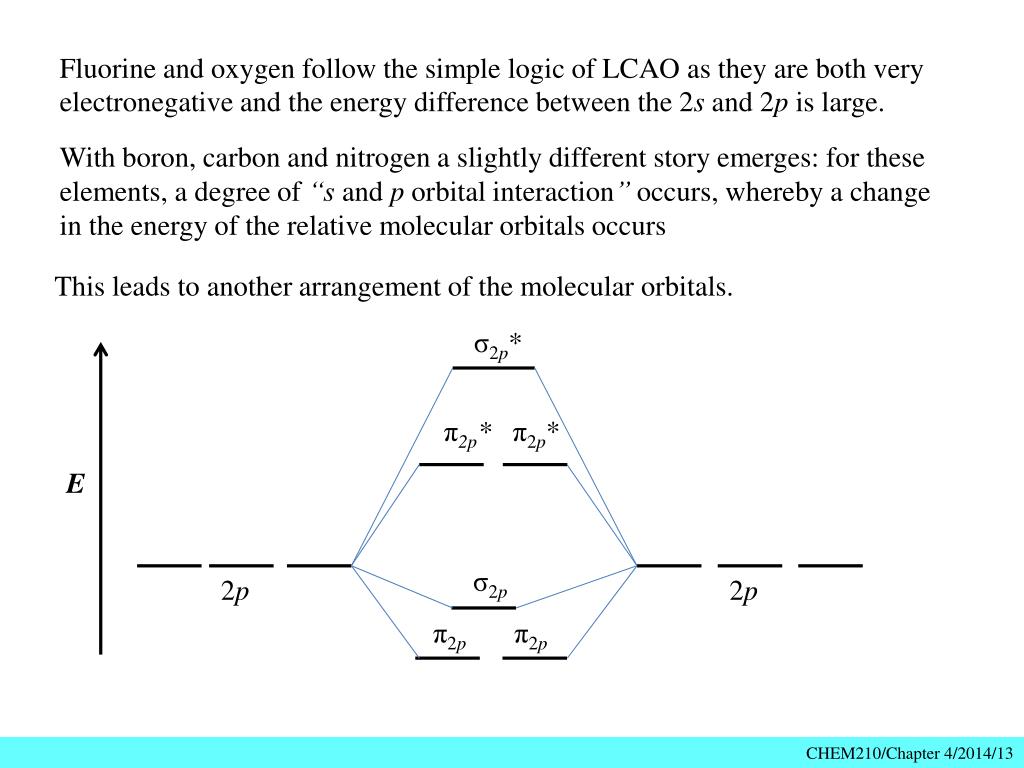
For lighter elements, such as Boron, carbon, nitrogen, the π2py and π2pzare less in energy than the σ2px and the σ*2px is lesser in energy than the π*2py and π*2pz orbitals. For these atoms the order is; σ1s, σ*1s, σ2s, σ*2s,(π2py,π2pz), σ2px,σ*2px, (π*2py,π*2pz). The molecular orbital diagrams for molecules and ions are drawn ...
Imaging the atomic orbitals of ... Things to note: 1) The color of the liquid oxygen; 2) Molecular orbital diagrams for nitrogen and for oxygen.
Tagged as: #digitalkemistry, Bond order of Nitrogen molecule, chemical bonding, chemical bonding and shapes of molecules, chemistry, class 11 chemistry molecular orbital theory, class 11 chemistry molecular orbital theory notes, class 11 chemistry notes, digital kemistry, How do you draw a molecular orbital diagram, How do you find the bond ...
If you are still not getting the Nitrogen Electron Configuration of the element nitrogen then, the full electronic configuration of nitrogen is written as the following; 1s 2 2s 2 2p 3. If we gave you brief information then, the first two electrons lie in the 1s orbital, following the next 2 electrons, it comes under the 2s orbital.
Draw the molecular orbital diagram for n2 ion and calculate the bond order. One is for the elements up to nitrogen. The molecular orbital theory mo has been introduced for the diatomic hydrogen molecules. N2 molecular orbital diagram with nitrogen we see the two molecular orbitals mixing and the energy repulsion.
Explain the formation of nitrogen molecule by molecular orbital theory (MOT) . - Get the answer to this question by visiting BYJU'S Q&A Forum.
Molecular orbital diagram for ne2. Answer to draw the molecular orbital diagram for ne2 and determine if the bond between the two atoms will be stable. Give each mo an appropriate label. For ne2 construct three molecular orbital diagrams one each for the neutral molecule the 1 cation and the 1 anion. The other is for after nitrogen starting at.
My question is asking to describe the bonding in the NO2- ion using valnece bond theory versus Molecular Orbital theory. I know that the ion is bent and that there are 2 sigma bonds and 1 pi bond and that the nitrogen is sp2 hybridized. But the latter part of my question asks how MO theory describes the pi bond in the species. This is a general chem course and I frankly have no clue how to go about drawing a MO diagram for a non-diatomic molecule, so I’m guessing there’s something else I have...
Let me explain the molecular orbital diagram of n2 using its diagram. ... Molecular orbitals are formed by linear combination of atomic orbitals.
The molecular orbitals are listed in a column down the center of the diagram. ... orbital on the diagram displays the isosurface for that orbital in ...
Give The Molecular Orbital Energy Diagram Of N2 And O2 Write The Bond Order Of N2 And O2 Sarthaks Econnect Largest Online Education Community
The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 8). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one ...
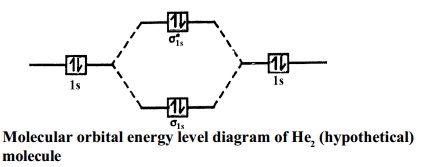
Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron.
December 9, 2015 - The change of the molecular orbital ordering between nitrogen and oxygen is the manifestation of this decreased s-p mixing. In the dioxygen molecular orbital scheme the s-p mixing effect is no longer significant enough to alter the relative orbital arrangement.

Write The Molecular Orbital Diagram Of N2 And Calculate Their Bond Order Chemistry Topperlearning Com Qbqjy
With nitrogen, we see the two molecular orbitals mixing and the energy repulsion. This is the reasoning for the rearrangement from a more familiar diagram. Notice how the σ from the 2p behaves more non-bonding like due to mixing, same with the 2s σ. This also causes a large jump in energy ...
Can you provide any evidence beyond a quick google search (i.e., literature) as to whether the M.O. diagram for nitrogen monoxide has the valence sigma and pi orbitals switched (e.g., as in the M.O. for molecular nitrogen) or is the sigma lower in energy than the pi orbitals?
Two p-atomic orbitals (one from each nitrogen) atom combine to form two molecular orbitals, the bonding molecular orbital σ2px and antibonding molecular orbital σ*2px. The other four p-atomic orbitals (two from each nitrogen) atom combine to give four molecular orbitals, two bonding molecular orbitals i.e. π2py and π2pz, while two ...
principal quantum number (n) → energy level in orbitals and its value could be any positive integer starting from 1 to infinity. The set of quantum numbers that is correct and consistent with n = 4 is (A) l = 3 m l = -3 m s = +1/2. What are the four quantum numbers of 1s1? 1.1.
Like nitrous oxide, it has a linear molecular structure, but with the great difference that the N = O bond also has the character of a triple bond. NO is rapidly oxidized in air to produce NO 2 , thus generating more stable molecular orbitals with a more oxidized nitrogen atom (+4). 2NO (g) + O 2 (g) => 2NO 2 (g)
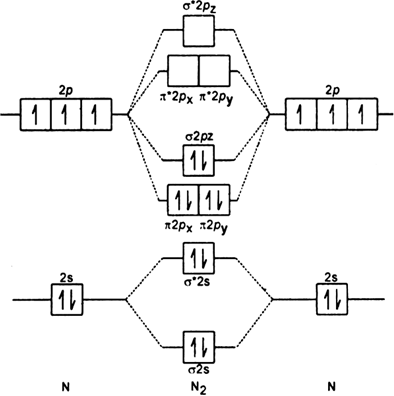
Discuss The Formation Of N2 Molecule On The Basis Of Mo Theory Predict Its I Bond Order Ii Magnetic Character From Chemistry Chemical Bonding And Molecular Structure Class 11 Haryana Board English Medium
The electronic configuration of nitrogen (Z=7) = 1s2 2s2 2px12py12pz1. The total number of electrons present in the nitrogen molecule (N2) is 14. In order to maximize energy, these 14 electrons can be accommodated in the different molecular orbitals.
There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc). One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start...
Draw and explain the molecular orbital diagram of carbon molecule. chemical bonding; class-11; Share It On Facebook Twitter Email. 1 Answer +1 vote . answered Dec 22, 2020 by Taashi (15.8k points) selected Dec 24, 2020 by Aashi01 . Best answer. 1. Electronic ...
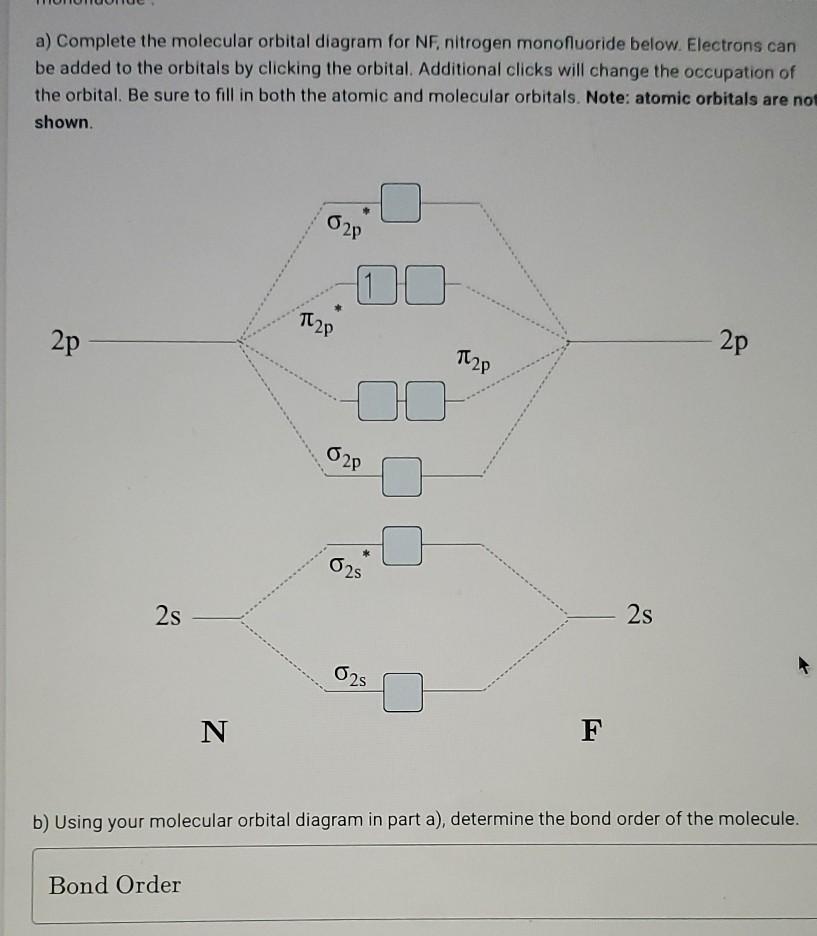
a) (3pt) The Molecular Orbital energy diagram of NO (nitrogen monoxide) is given in the right figure. ... Draw the molecular orbital diagram for B2.
Draw The Diagrams For No 2 No 2 And No 2 The Homo Of No 2 Shows It Is Somewhat Anti Bonding Would You Expect The Nonbonding Electron Pairs On Nitrogen Or Oxygen To Be More Reactive
August 12, 2020 - How? It's just the total number of valence electrons. For an example, we have used N2. Each nitrogen has five valence electrons, for a total of ten, so we have just filled in ten electrons, starting at the bottom of the molecular orbital energy level diagram.








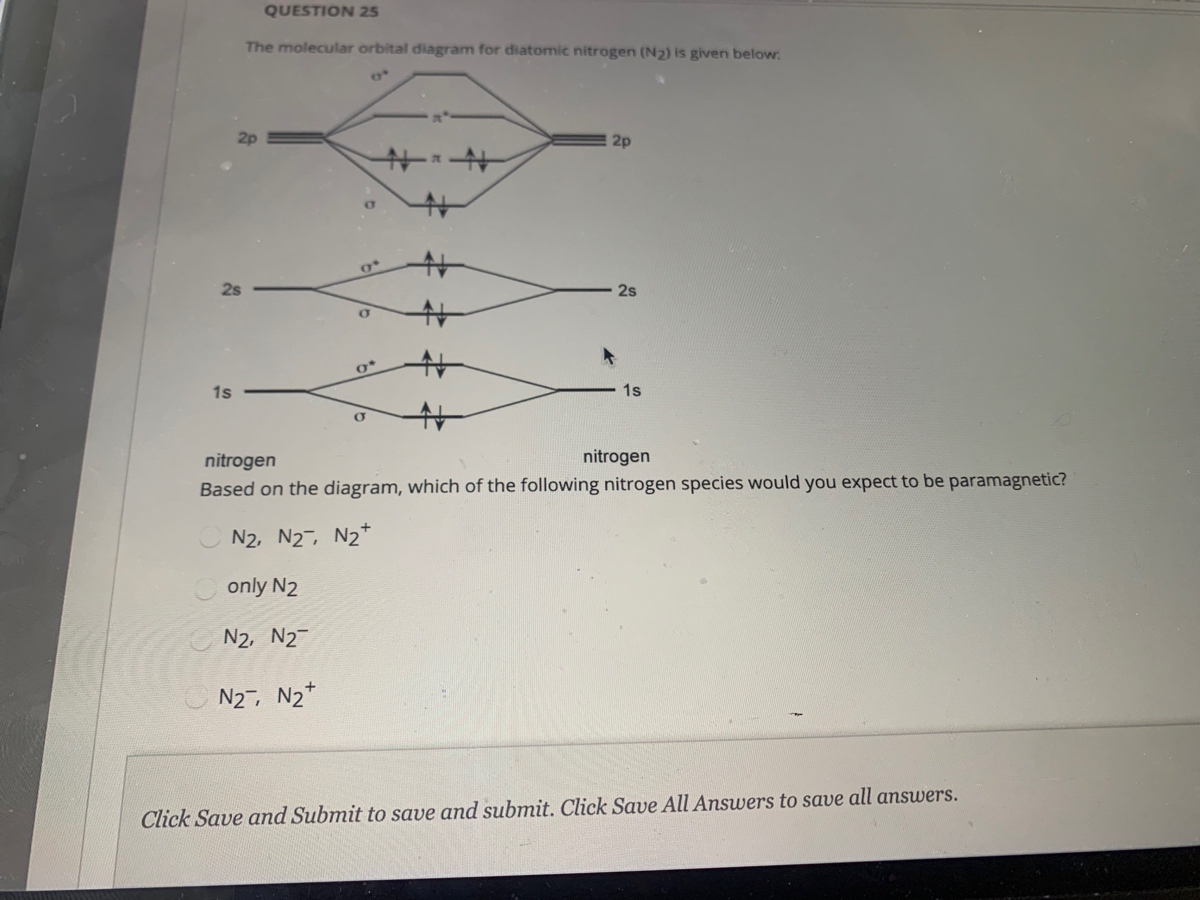




0 Response to "39 nitrogen molecular orbital diagram"
Post a Comment