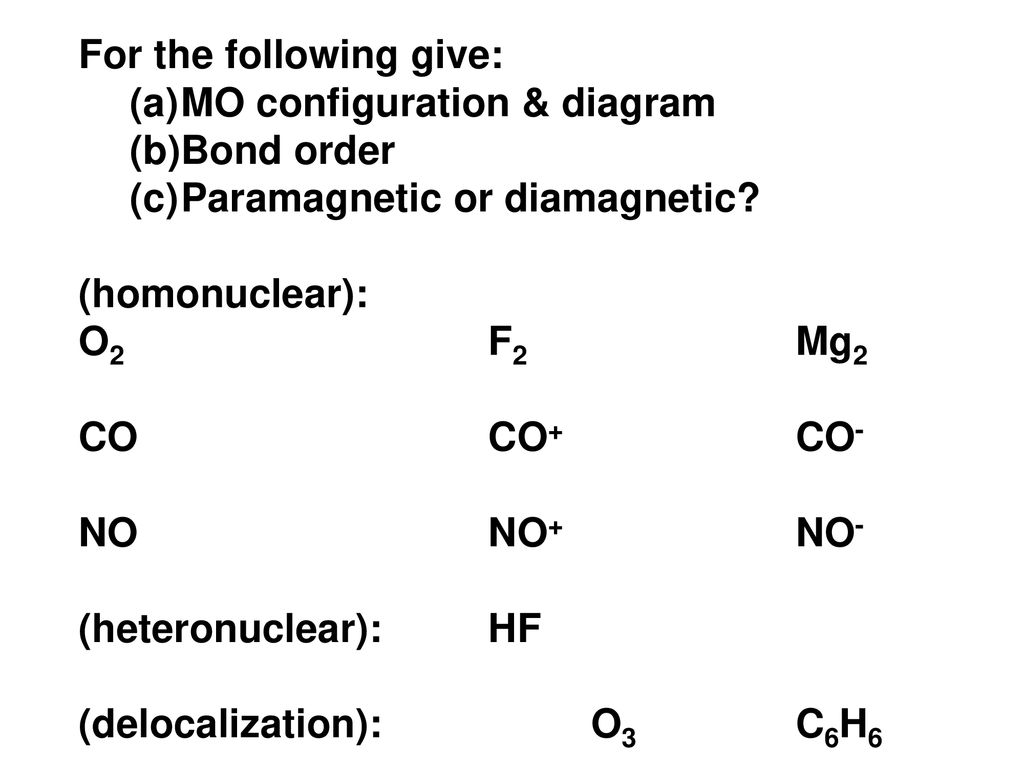
41 mg2 molecular orbital diagram
Magnesium Chloride (MgCl2) - BYJUS Magnesium Chloride. Molecular weight/molar mass of MgCl 2. 95.211 g/mol (anhydrous) Density of Magnesium Chloride. 2.32 g/cm 3 (anhydrous) Boiling Point of Magnesium Chloride. 1,412 °C. Melting Point of Magnesium Chloride. 714 °C. Cl2 Molecular Orbital Diagram A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.Second-Row Diatomic Molecules - Chemistry LibreTextsMolecular orbital diagram - Wikipedia
A molecular orbital study of a model ... - ScienceDirect.com Volume 179, Issue 1, October 1988, Pages 145-152 A molecular orbital study of a model of the Mg2+ coordination complex of the self splicing reaction of ribosomal RNA Dedicated to Professor Bernard Pullman. MaryMcCourtab MasayukiShibatab James W.McIverJr.ab RobertRein b (88)80119-2 Get rights and content Abstract
Mg2 molecular orbital diagram
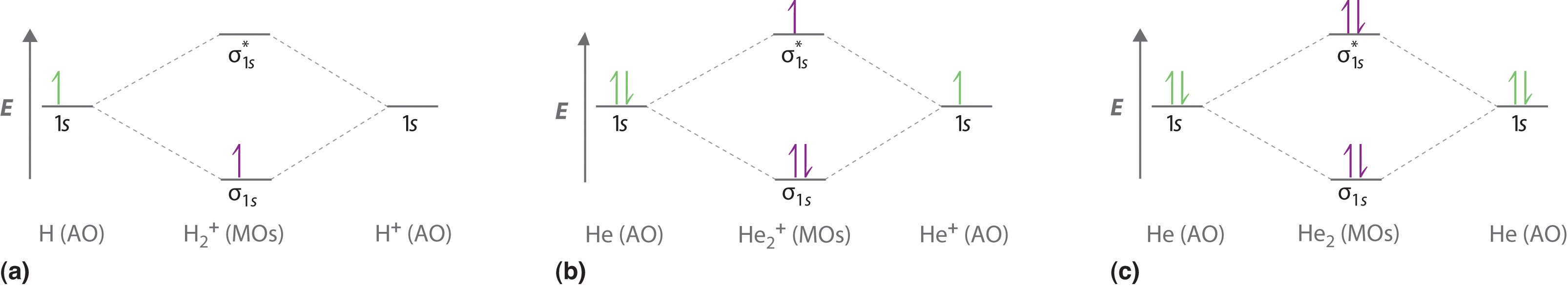
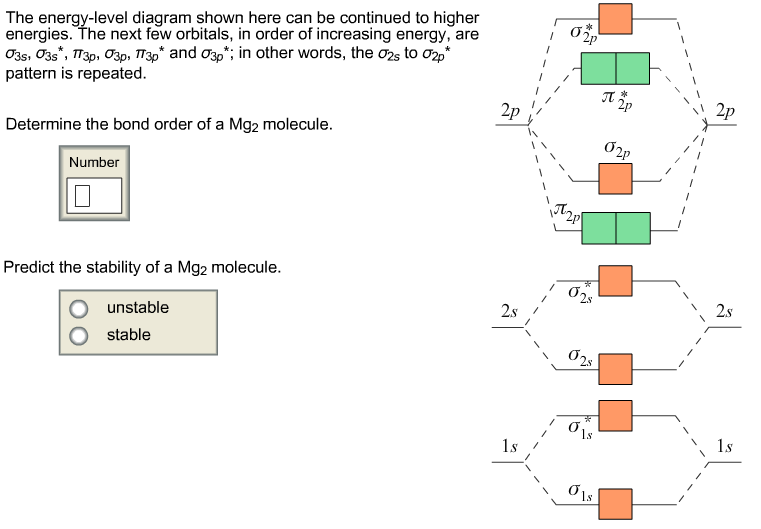
12.5: Molecular Orbital Theory - Chemistry LibreTexts We can now fill the molecular orbital diagram: The two electrons occupy the lowest-energy molecular orbital, which is the bonding (σ 1s) orbital, giving a (σ 1s) 2 electron configuration. To avoid violating the Pauli principle, the electron spins must be paired. C So the bond order is 2 − 0 2 = 1 PDF Molecular Orbital Approach to Bonding - Texas A&M University Solution will involve molecular orbitals - similar to atomic orbitals - but centred around all of the nuclei in molecule. Each defined by sets of quantum numbers, with electron probability density determined by ψ2, where ψ = molecular wave function. Approximate method: At any moment, electron near one nucleus - approximate behaviour like What is the bond order of Mg2? The number of bonding and non-bonding electrons can be determined using the molecular orbital (MO) theory, which suggests that there are two ways for the orbital interaction to occur between two...
Mg2 molecular orbital diagram. MO Diagrams - University of Sydney Molecular Orbital Diagram Maker. These quizzes enable you to build your own molecular orbital diagram from components. A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. Molecular Orbital Theory · Chemistry Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ... Orbital Diagram of All Elements (Diagrams given Below) Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15 ... What is the orbital diagram for magnesium? - Quora Which is the molecular orbital diagram for HF? The electronic of hydrogen and fluorine are 1s¹ and 1s²2s²2p⁵ respectively. In the formation of HF molecule ,only 2p electrons of fluorine atom would combine effectively with the solitary electron of hydrogen atom. As has been already explained ,only a pz orbital is able to combine with s orbital.
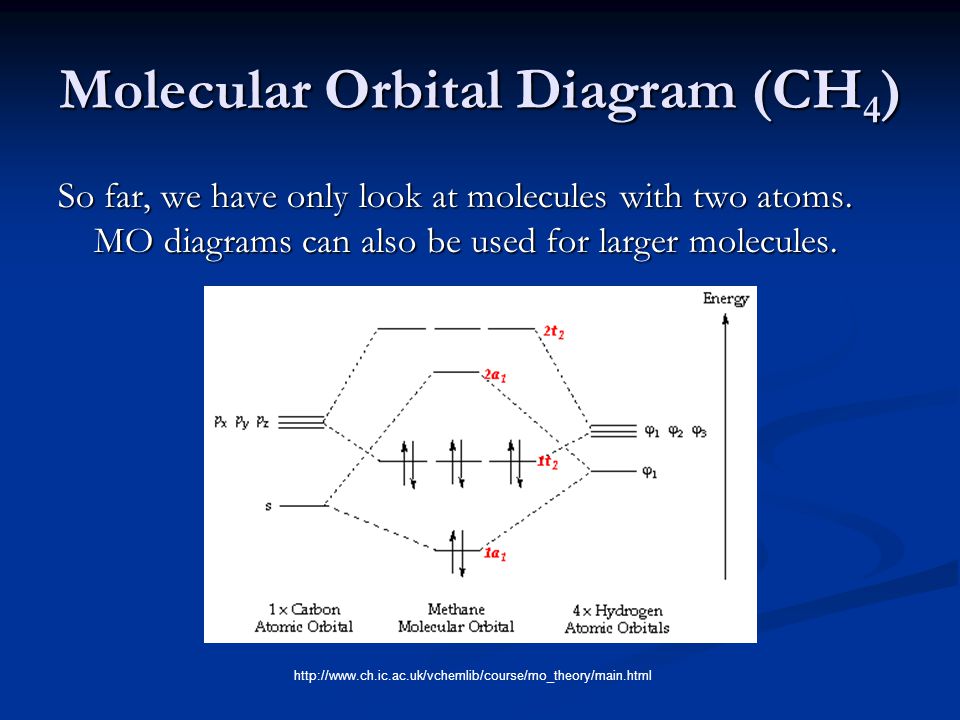
Molecular Orbital Theory - Lumen Learning Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals ( s, p, d …) and hybrid orbitals ( sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals ( σ σ, σ σ *, π π, π π *) What is the electron configuration of Mg 2 - AskingLot.com Moreover, what is the electron configuration of mg2+? The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the remaining two electrons in the 3s. Therefore the Magnesium electron configuration will be 1s22s22p63s2. Also Know, what does mg2+ represent? Mg+2 - PubChem Magnesium ion is a natural product found in Phytelephas aequatorialis, Montanoa frutescens, and other organisms with data available. Magnesium cation is a Calculi Dissolution Agent and Osmotic Laxative. The mechanism of action of magnesium cation is as a Magnesium Ion Exchange Activity and Osmotic Activity. The physiologic effect of magnesium ... 3.3.4: Assembling a complete MO diagram - Chemistry LibreTexts A molecular orbital interaction diagram shows how atomic or molecular orbitals combine together to make new orbitals. Sometimes, we may be interested in only the molecular orbital energy levels themselves, and not where they came from. A molecular orbital energy level diagram just shows the energy levels in the molecule.
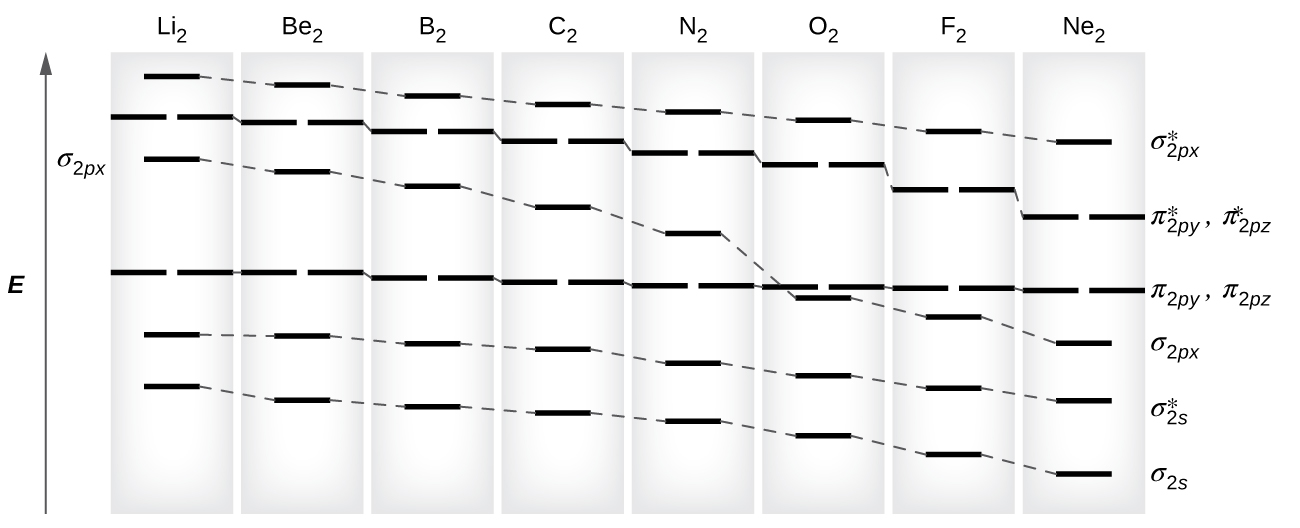
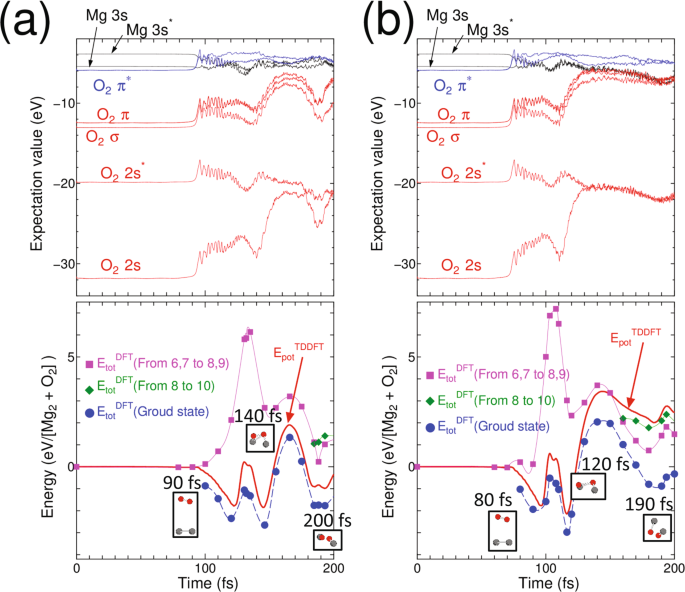
Schematic molecular orbital diagrams of Al 2 and AlSi. For ... Download scientific diagram | Schematic molecular orbital diagrams of Al 2 and AlSi. For simplic- from publication: Electronic structure and photoelectron spectroscopy of AlSi mixed dimer | The ... Molecular Orbital (MO) Diagram of O2 - YouTube Molecular Orbital Diagram for Oxygen Gas (O2).Fill from the bottom up, with 12 electrons total.Bonding Order is 2, and it is Paramagnetic.sigma2s(2),sigma2s*... Molecular Orbital MO Diagram for N2(2-) - YouTube the pi(2p) bonding orbitals are LOWER than the sigma(2p) bonding orbitals.N2(2-) has a bonding order of 2, which predicts that there will be a stable double ... Electronic Potential Energy Curves of the Mg2 ... - NIST Potential curve for Mg 2 calculated within a basis of 56 contracted Gaussian type orbitals (the calculation of the correlation energy used 54 molecular orbitals since the 1σ g and 1σ u orbitals were frozen). Reference Figure 4. Comparison of theoretical and experimental (RKR) van der Waals wells for the X 1 Σ g+ state of Mg 2.
What is the electronic configuration of Mg ... - Socratic.org The electron configuration of the magnesium ion Mg2+ is similar to Na+, Ne, and Al3+. It is not similar to Ar. Okay, so we are given these answer choices, but how do we know which 3 share the same electron configuration as Mg2+? Before going on, it is important to note that when an element is written as Mg2+, it means that it is losing 2 electrons.
A molecular orbital study of a model of ... - ScienceDirect Volume 179, Issue 1, October 1988, Pages 145-152 A molecular orbital study of a model of the Mg2+ coordination complex of the self splicing reaction of ribosomal RNA Dedicated to Professor Bernard Pullman. MaryMcCourtab MasayukiShibatab James W.McIverJr.ab RobertRein b (88)80119-2 Get rights and content Abstract
Schupf Computational Chemistry Lab - Colby MG2 - O3 - H4 Tell me about the atomic charges, dipole moment, bond lengths, angles, bond orders, molecular orbital energies, or total energy. Tell me about the best Lewis structure. Atomic Charges and Dipole Moment O1 charge=-1.046 MG2 charge= 1.256 O3 charge=-1.045 H4 charge= 0.416 H5 charge= 0.418 with a dipole moment of 0.01372 Debye
What is the molecular orbital diagram for C ... - Socratic.org The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4. The electron configuration of the C− 2 ion will be.
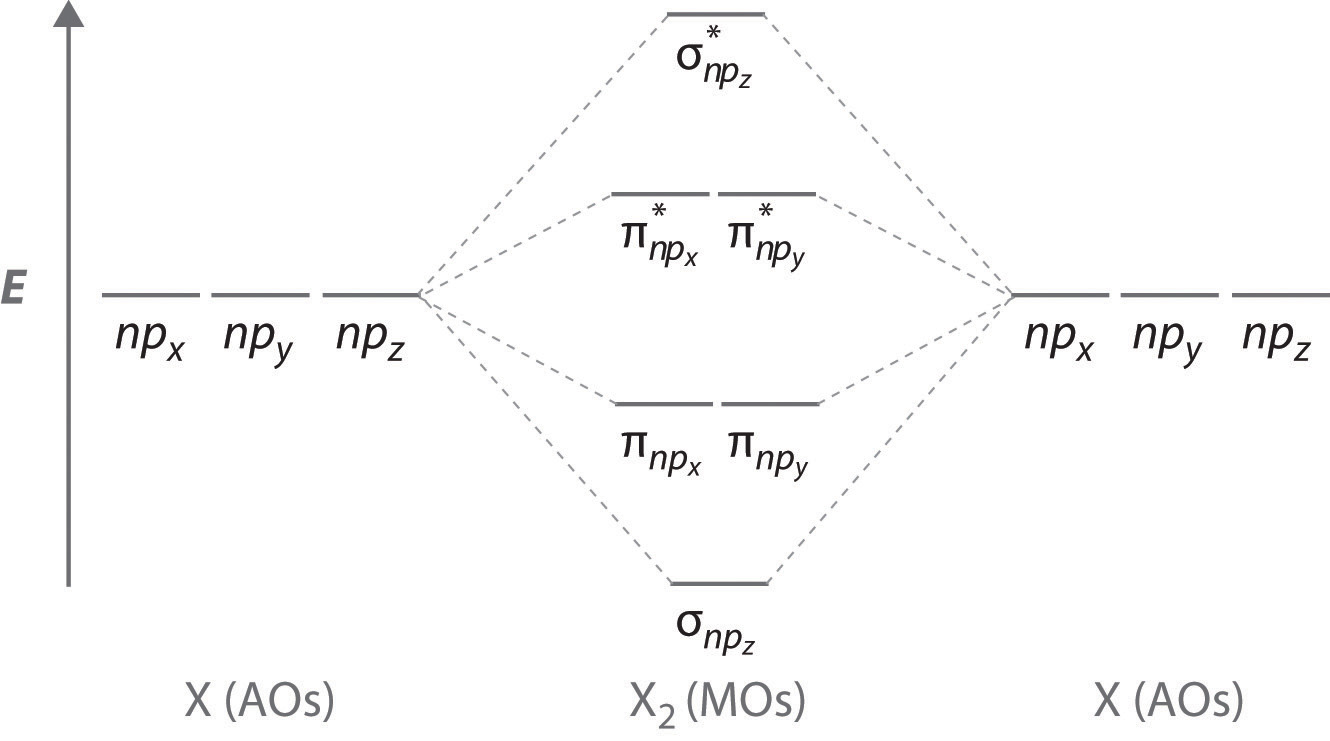
Molecular Orbital Theory Molecular orbital diagrams are diagrams of MO energy levels, shown as short horizontal lines in the center. Atomic orbitals (AO) energy levels are shown for comparison. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels. Levels with the same energy are commonly shown side by side.
PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
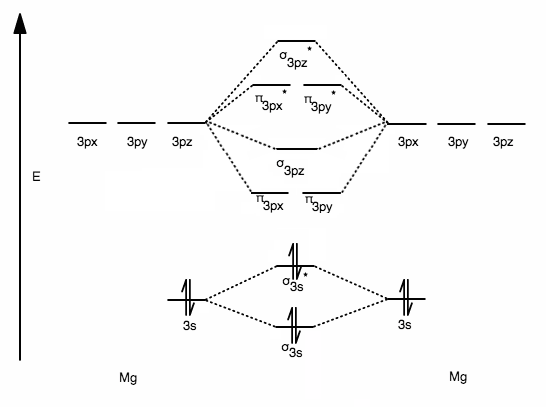
Solved 13. Draw the molecular orbital diagram for ... - Chegg 13. Draw the molecular orbital diagram for the molecule Mg2. Determine the bond order of the molecule and indicate whether or not the molecule exists. Question: 13. Draw the molecular orbital diagram for the molecule Mg2. Determine the bond order of the molecule and indicate whether or not the molecule exists.
Molecular orbital The qualitative approach of MO analysis uses a molecular orbital diagram to visualize bonding interactions in a molecule. In this type of diagram, the molecular orbitals are represented by horizontal lines; the higher a line the higher the energy of the orbital, and degenerate orbitals are placed on the same level with a space between them.
MO Diagrams - GitHub Pages Molecular Orbital (MO) Theory is the final theory pertaining to the bonding between molecules. In contrast to VSEPR and valence bond theory which describe bonding in terms of atomic orbitals, molecular orbital theory visualizes bonding in relation to molecular orbitals, which are orbitals that surround the entire molecule. The purpose of MO theory is to fill in the gap for some behavior that ...
PDF Mg2 molecular orbital diagram What is molecular orbital diagram. n¡Ãmi le rop sad Ãarta netneis es euq sacit©Ãngamarap sartseum sal ,)2 arugiF( ocit©Ãngam opmac nu ne odidem osep la artseum anu ed osep le somarapmoc odnauC .n³Ãinu ed latibro nu ne etreivnoc al euq ol ,sotnuj somot¡Ã sod sol renetnam a naduya y soelcºÃn sobma noc naºÃtcaretni latibro etse ne ...
8.4 Molecular Orbital Theory This is the molecular orbital diagram for the homonuclear diatomic Be 2+, showing the molecular orbitals of the valence shell only. The molecular orbitals are filled in the same manner as atomic orbitals, using the Aufbau principle and Hund's rule. Bond Order
What is the bond order of Mg2? The number of bonding and non-bonding electrons can be determined using the molecular orbital (MO) theory, which suggests that there are two ways for the orbital interaction to occur between two...
PDF Molecular Orbital Approach to Bonding - Texas A&M University Solution will involve molecular orbitals - similar to atomic orbitals - but centred around all of the nuclei in molecule. Each defined by sets of quantum numbers, with electron probability density determined by ψ2, where ψ = molecular wave function. Approximate method: At any moment, electron near one nucleus - approximate behaviour like
12.5: Molecular Orbital Theory - Chemistry LibreTexts We can now fill the molecular orbital diagram: The two electrons occupy the lowest-energy molecular orbital, which is the bonding (σ 1s) orbital, giving a (σ 1s) 2 electron configuration. To avoid violating the Pauli principle, the electron spins must be paired. C So the bond order is 2 − 0 2 = 1




![Linear group 13 E[triple bond, length as m-dash]E triple ...](https://pubs.rsc.org/image/article/2021/CP/d1cp01035b/d1cp01035b-s1_hi-res.gif)
















0 Response to "41 mg2 molecular orbital diagram"
Post a Comment