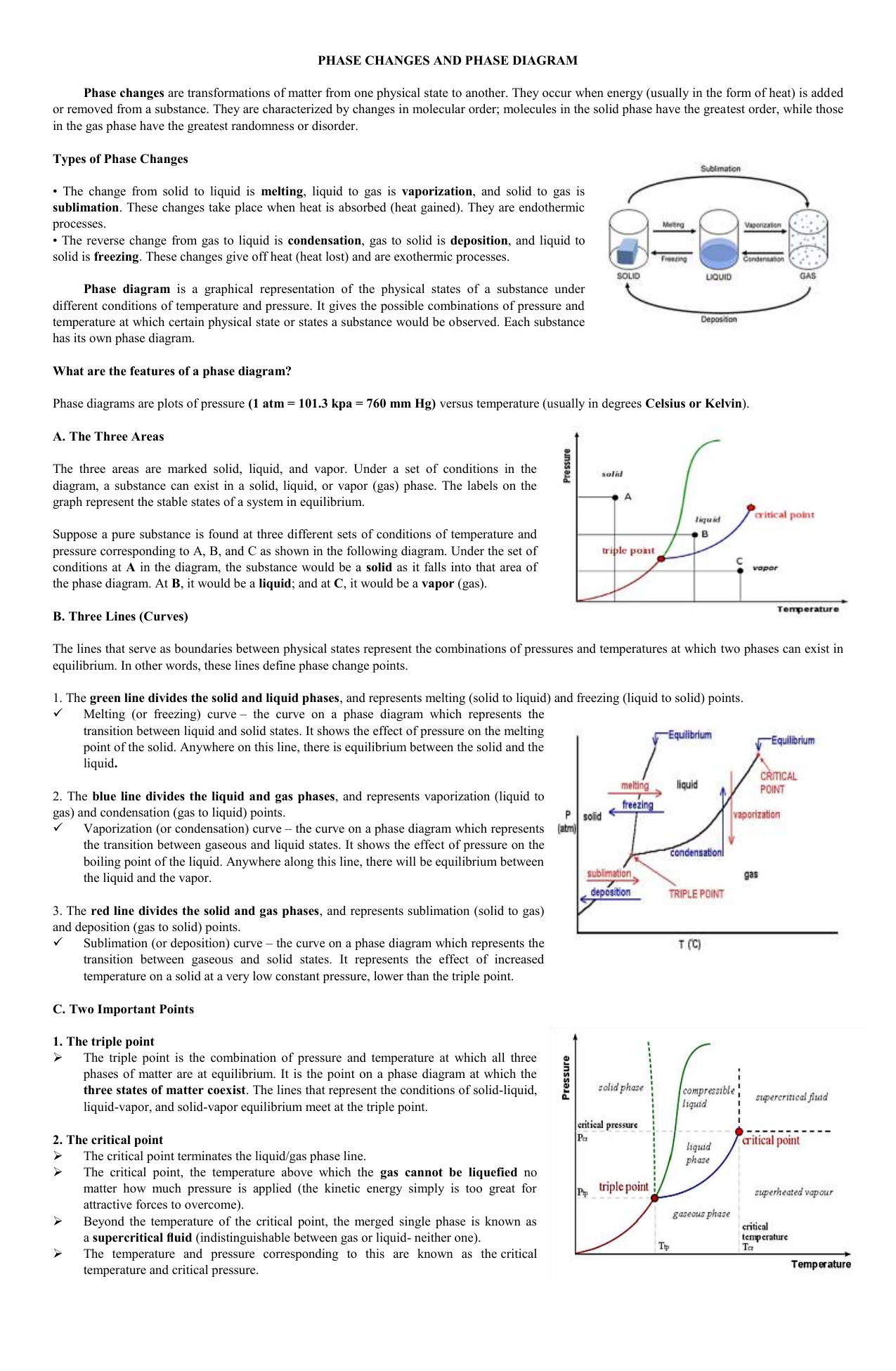
41 in this phase diagram for water indicate the direction that the solid-liquid and liquid-gas
12.4: Phase Diagrams - Chemistry LibreTexts In contrast to the phase diagram of water, the phase diagram of CO 2 (Figure \(\PageIndex{3}\)) has a more typical melting curve, sloping up and to the right. The triple point is −56.6°C and 5.11 atm, which means that liquid CO 2 cannot exist at pressures lower than 5.11 atm. Phase Diagrams - Phases of Matter and Phase Transitions A phase diagram for water might include the temperatures and pressures at which ice forms orthorhombic and hexagonal crystals. A phase diagram for an organic compound could include mesophases, which are intermediate phases between a solid and a liquid. Mesophases are of particular interest for liquid crystal technology.
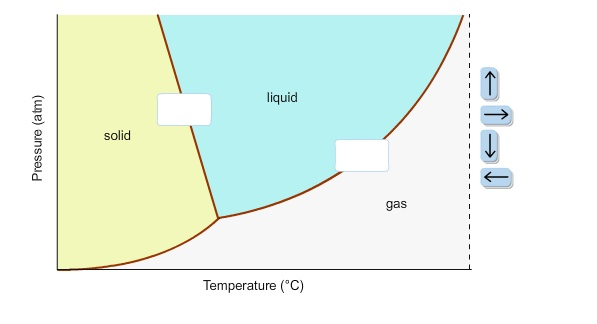
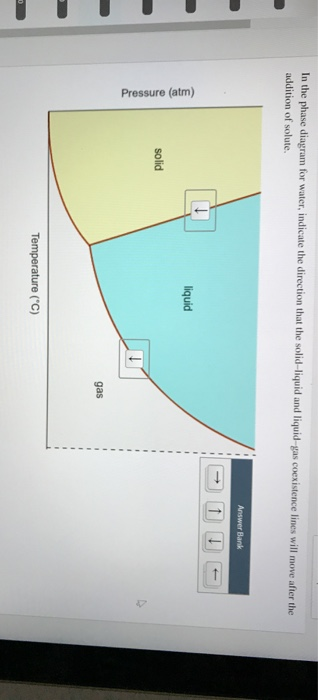
Solved In the phase diagram for water, indicate the ... Question: In the phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move along the temperature axis after the addition of solute. Answer Bank + liquid Pressure (atm) solid gas Temperature (°C) Vitamin K is involved in normal blood clotting.
In this phase diagram for water indicate the direction that the solid-liquid and liquid-gas
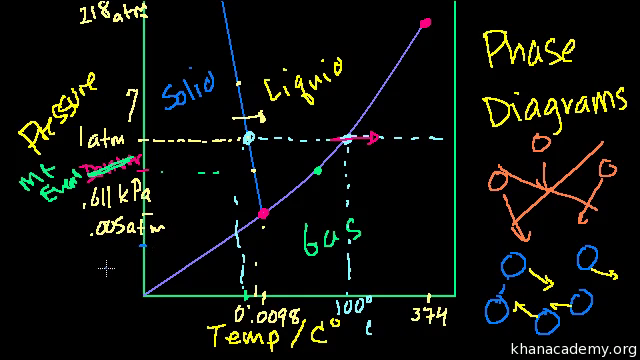
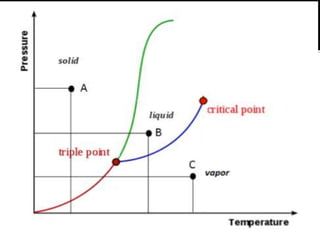
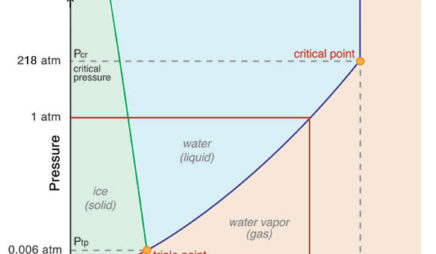
Phase diagram of water in real life | Physics Forums The phase diagrams of water and carbon dioxide indicate only states (P , V) of pressure and temperature that are in thermodynamical equilibrium but in real life, both water and dry ice are not in equilibrium: water is present both as a liquid (in oceans and lakes) and as a vapor at the same conditions of pressure P and temperature T. Phase diagram - Wikipedia The simplest phase diagrams are pressure-temperature diagrams of a single simple substance, such as water.The axes correspond to the pressure and temperature.The phase diagram shows, in pressure-temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas.. The curves on the phase diagram show the points where the free energy (and other ... Phase Diagrams - Chemistry LibreTexts The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to liquid). There are also two important points on the diagram, the triple point and the critical point.
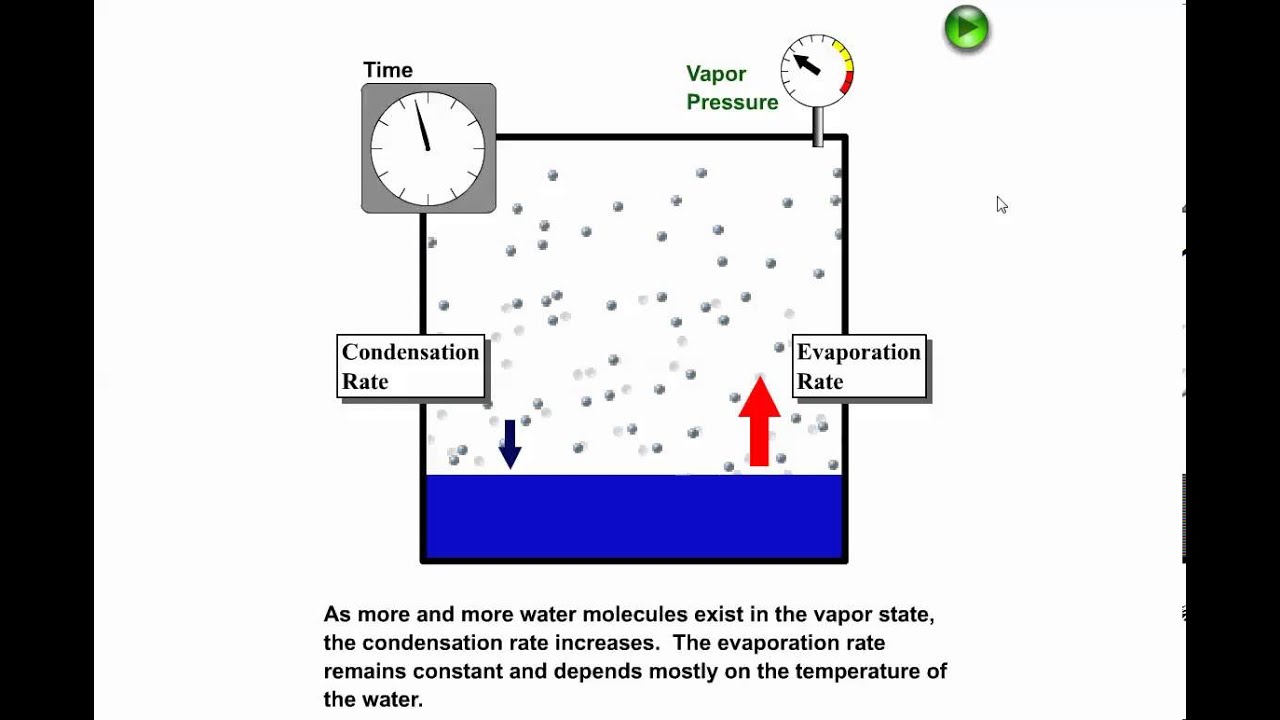
In this phase diagram for water indicate the direction that the solid-liquid and liquid-gas. OneClass: In this phase diagram for water, indicate the ... In this phase diagram for water, indicate the direction that thesolid-liquid and liquid-gas coexistence lines will move after theaddition of solute. In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. Solved In this phase diagram for water, indicate the ... Expert Answer 100% (60 ratings) when solute is added to water it will decrease the freezing point of the water and increase the boiling point of the wa … View the full answer Transcribed image text: In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. PDF Phase diagram of water - Columbia University Phase diagram of water Note: for H2O melting point decreases with increasing pressure, for CO2 melting point increases with increasing pressure. ... Melting point - temperature at which a substance turns from solid to liquid. solid liquid gas. The three phase changes can be brought about by changes in temperature or pressure: Answered: In the phase diagram for water,… | bartleby Answer Bank liquid solid gas Temperature (°C) Pressure (atm) Question Transcribed Image Text: In the phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move along the temperature axis after the addition of solute. Answer Bank liquid solid gas Temperature (°C) Pressure (atm) Expert Solution
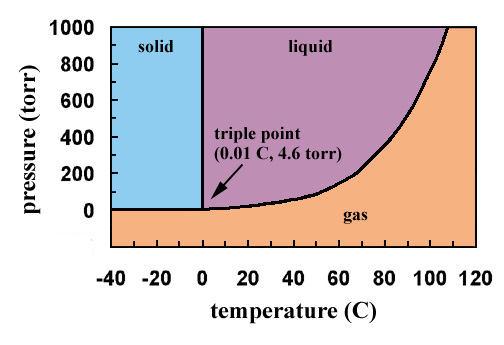
Solved In the phase diagram for water, indicate the ... Answer Bank liquid Pressure (atm) solid gas Temperature (°C) Question: In the phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. Answer Bank liquid Pressure (atm) solid gas Temperature (°C) phase diagrams of pure substances - chemguide Imagine lowering the pressure on liquid water along the line in the diagram below. The phase diagram shows that the water would first freeze to form ice as it crossed into the solid area. When the pressure fell low enough, the ice would then sublime to give water vapour. In other words, the change is from liquid to solid to vapour. Phase Diagrams - GitHub Pages The line that connects points A and C is the vapor pressure curve of the solid phase. Along this line, the solid is in equilibrium with the vapor phase through sublimation and deposition. Finally, point A, where the solid/liquid, liquid/gas, and solid/gas lines intersect, is the triple point HW2 - Homework Answers - Google Search In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. 15. Menthol is a crystalline substance with a...
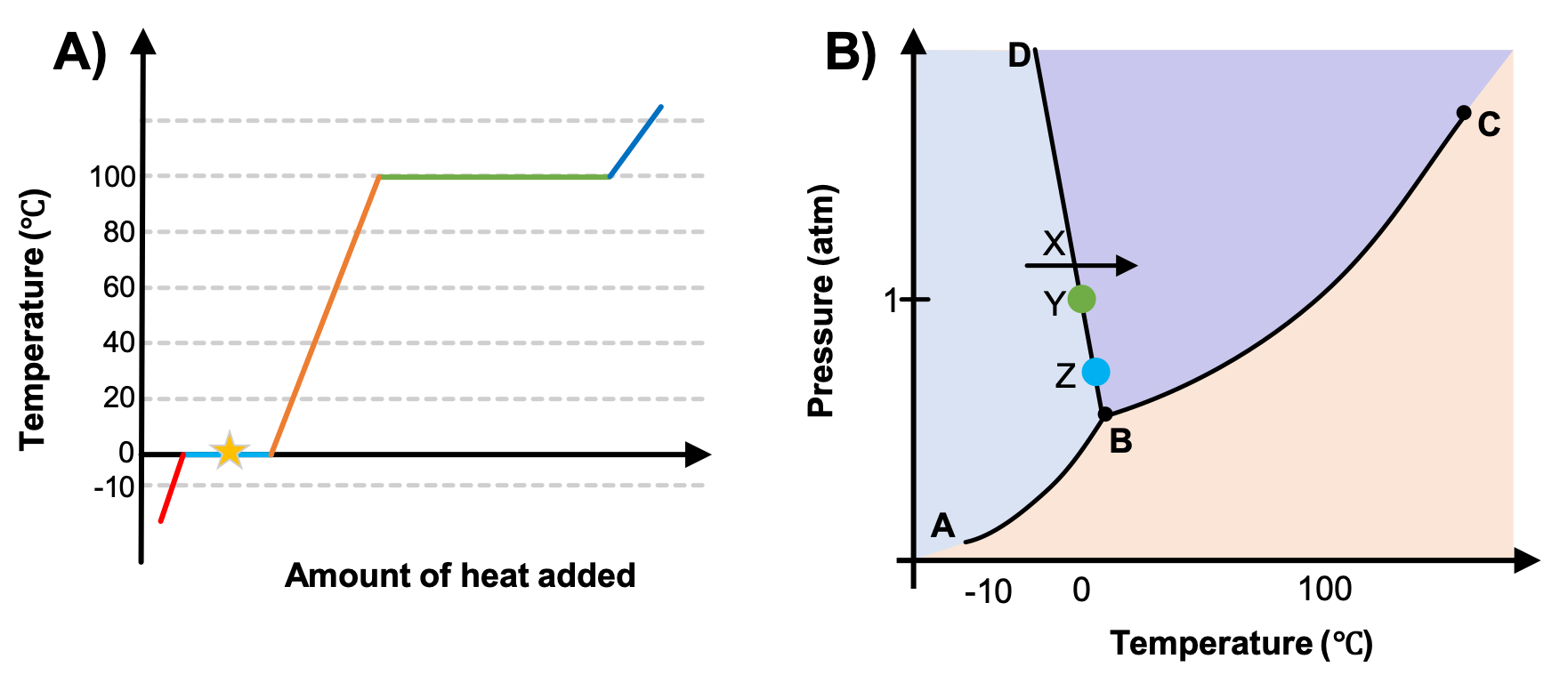
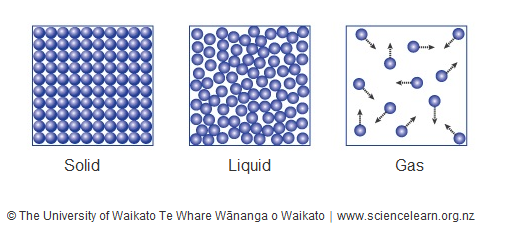
Chem Sapling Hw Ch 14 Flashcards - Quizlet In this diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. Solid <----- Liquid Liquid ------> Gas 10. A solution is made by dissolving 0.617 mol of nonelectrolytes solute in 889 g of benzene. Calculate the freezing point, Tf, and boiling point Tb of the solution. Gas To Phase Solid Change [N1BU6A] What is Gas To Solid Phase Change. phase change - a change from one state (solid or liquid or gas) to another without a change in chemical composition. below the boiling point of the substance) then we call this a vapour phase. Water vapor has the highest internal energy per gram of water, followed by liquid water, and then ice. Chemistry 1 Exam Flashcards - Quizlet Classify each phase change based on whether it describes a transition between a gas and a liquid, a gas and a solid, or a liquid and a solid Gas and liquid -condensation -evaporation Gas and solid -sublimation -deposition Liquid and solid -freezing -melting Label the heating curve with the phase or phases present. Assume constant pressure 10.4 Phase Diagrams - Chemistry Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ...
Phase Diagram for Water | Chemistry for Non-Majors In water's diagram, the slope of the line between the solid and liquid states is negative rather than positive. The reason is that water is an unusual substance in that its solid state is less dense than the liquid state. Ice floats in liquid water. Therefore, a pressure change has the opposite effect on those two phases.
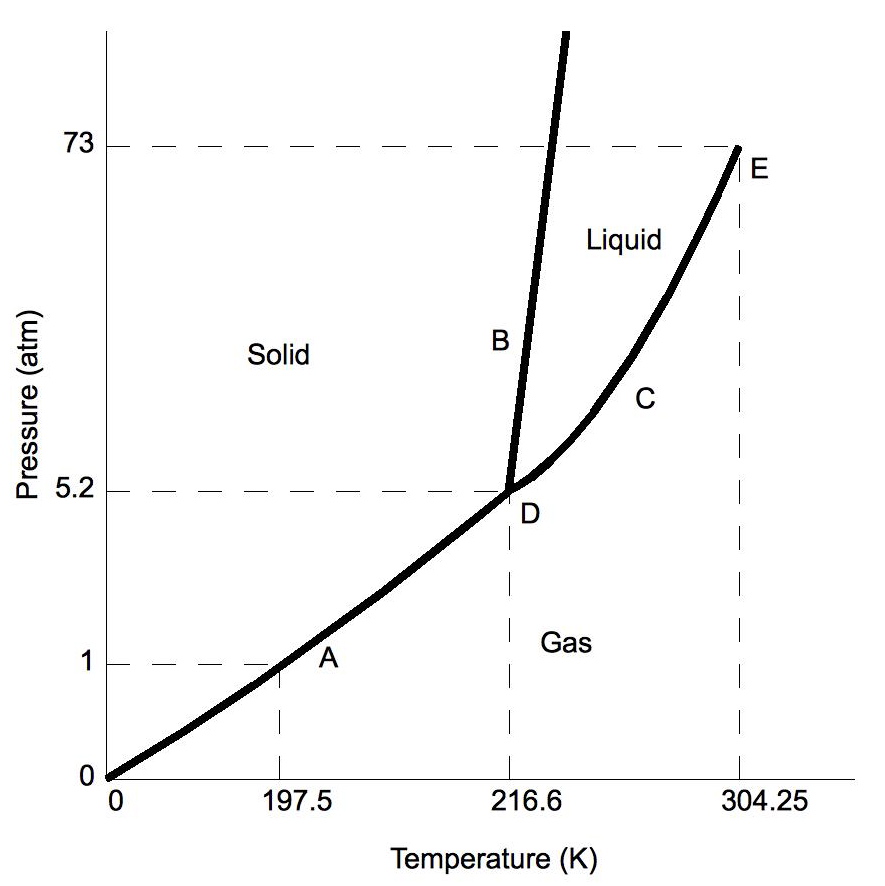
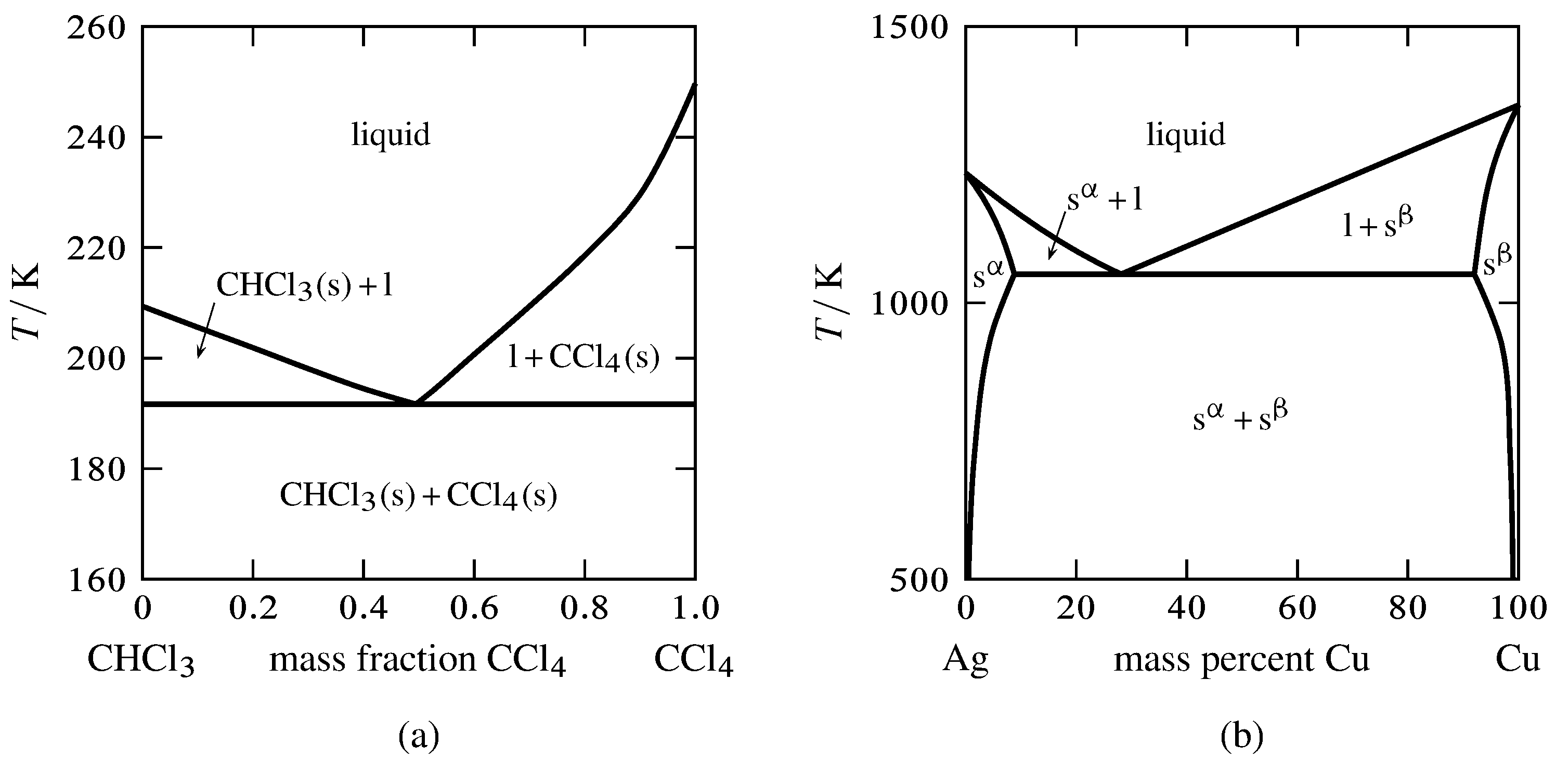
Chapter 7.7: Phase Diagrams - Chemistry LibreTexts Figure 7.7.2 Two Versions of the Phase Diagram of Water (a) In this graph with linear temperature and pressure axes, the boundary between ice and liquid water is almost vertical. (b) This graph with an expanded scale illustrates the decrease in melting point with increasing pressure. (The letters refer to points discussed in Example 10.)
Solved In the phase diagram for water, indicate ... - Chegg Science Chemistry Chemistry questions and answers In the phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move along the temperature axis after the addition of solute. Answer Bank 1. → liquid Pressure (atm) solid gas Temperature (°C)
Phase Diagrams - Purdue University The phase diagram for water is shown below. The solid lines identify the temperatures and pressures at which an equilibrium exist between phases. The point at which the lines intersect represents the triple point. At the pressure and temperature of the triple point, all three phases (solid, liquid and gas) exist in equilibrium. The triple point ...
In this phase diagram for water, indicate the direction ... In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. In this phase diagram for water, indicate the direction that the solid-liquid and liquid-gas coexistence lines will move after the addition of solute. Expert's Answer Solution.pdf Next Previous
11.7: Phase Diagrams - Chemistry LibreTexts The Phase Diagram of Carbon Dioxide. In contrast to the phase diagram of water, the phase diagram of CO 2 (Figure 11.7.3 ) has a more typical melting curve, sloping up and to the right.The triple point is −56.6°C and 5.11 atm, which means that liquid CO 2 cannot exist at pressures lower than 5.11 atm. At 1 atm, therefore, solid CO 2 sublimes directly to the vapor while maintaining a ...
Phase (matter) - Wikipedia This unusual feature of water is related to ice having a lower density than liquid water. Increasing the pressure drives the water into the higher density phase, which causes melting. Another interesting though not unusual feature of the phase diagram is the point where the solid-liquid phase line meets the liquid-gas phase line.
In this phase diagram for water, indicate the direction ... A phase diagram is a graph of pressure versus temperature depicting the solid, liquid and gaseous phases of a single substance under different conditions of temperature and pressure. In the phase diagram of water, the solid-liquid coexistence line indicates the point at which the solid converts to liquid. The temperature corresponding to
Phase Diagrams - Chemistry - University of Hawaiʻi The solid-liquid curve labeled BD shows the temperatures and pressures at which ice and liquid water are in equilibrium, representing the melting/freezing points for water. Note that this curve exhibits a slight negative slope (greatly exaggerated for clarity), indicating that the melting point for water decreases slightly as pressure increases.
Lecture 13: Introduction to the thermodynamics of phase ... The water phase diagram is shown in the figure below: Here we see the slight slope to the left of the solid-liquid coexistence line. As with benzene, the solid region of the phase diagram of water also exhibits a rich variety of different structures, the ice polymorphs, not shown in the phase diagram above.
Phase Diagrams - Chemistry LibreTexts The green line divides the solid and liquid phases and represents melting (solid to liquid) and freezing (liquid to solid). The blue divides the liquid and gas phases, represents vaporization (liquid to gas) and condensation (gas to liquid). There are also two important points on the diagram, the triple point and the critical point.
Phase diagram - Wikipedia The simplest phase diagrams are pressure-temperature diagrams of a single simple substance, such as water.The axes correspond to the pressure and temperature.The phase diagram shows, in pressure-temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas.. The curves on the phase diagram show the points where the free energy (and other ...
Phase diagram of water in real life | Physics Forums The phase diagrams of water and carbon dioxide indicate only states (P , V) of pressure and temperature that are in thermodynamical equilibrium but in real life, both water and dry ice are not in equilibrium: water is present both as a liquid (in oceans and lakes) and as a vapor at the same conditions of pressure P and temperature T.










:max_bytes(150000):strip_icc()/phase-changes-56a12ddd3df78cf772682e07.png)


0 Response to "41 in this phase diagram for water indicate the direction that the solid-liquid and liquid-gas"
Post a Comment