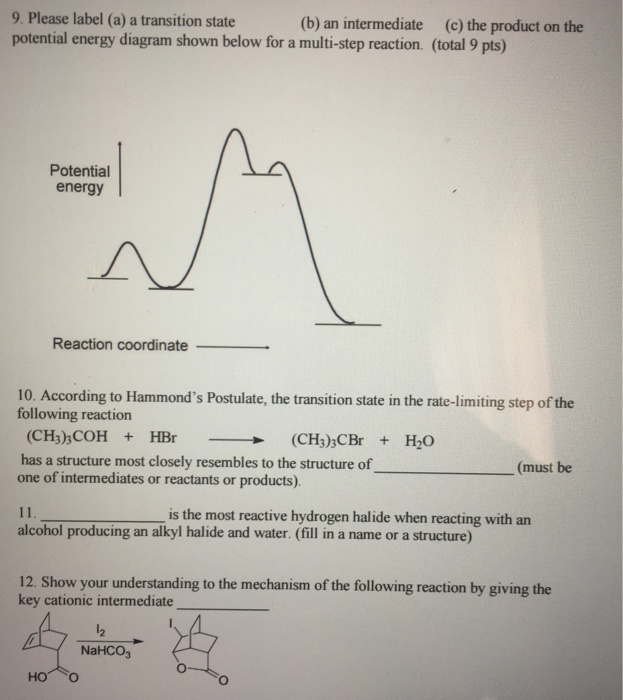
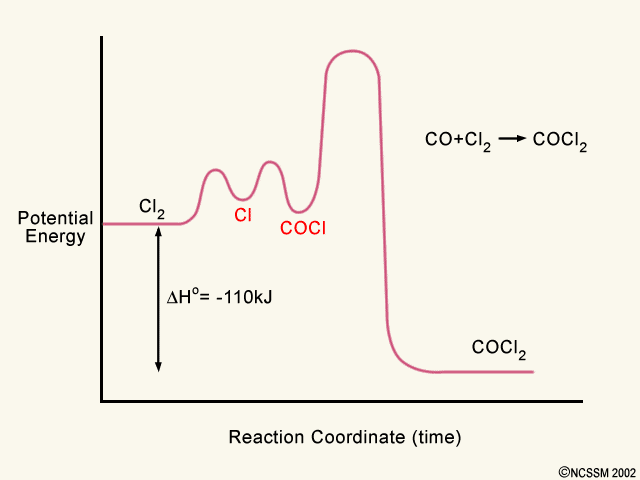
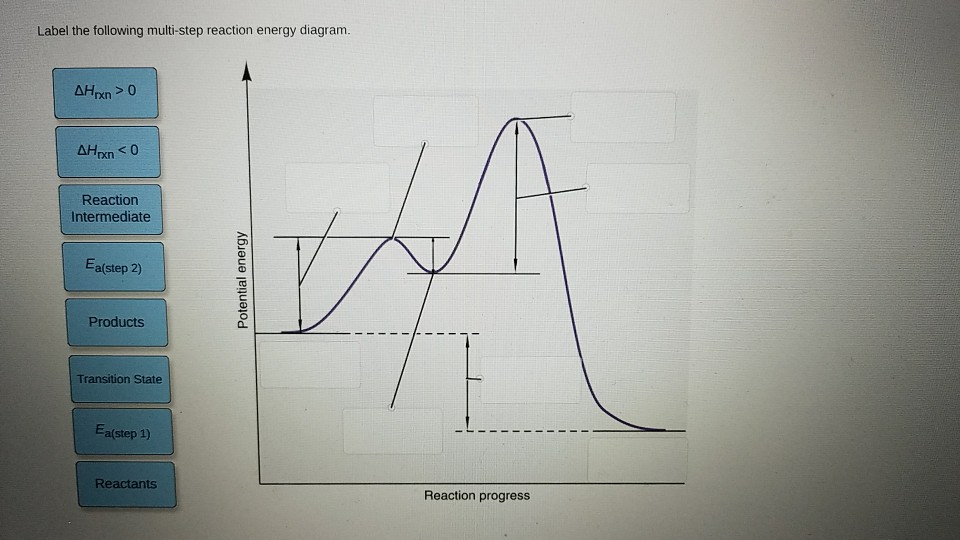
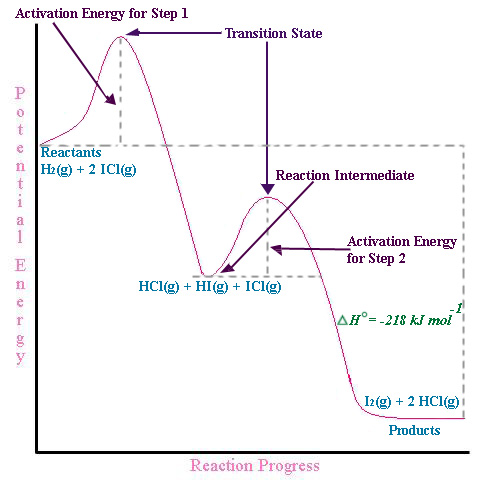
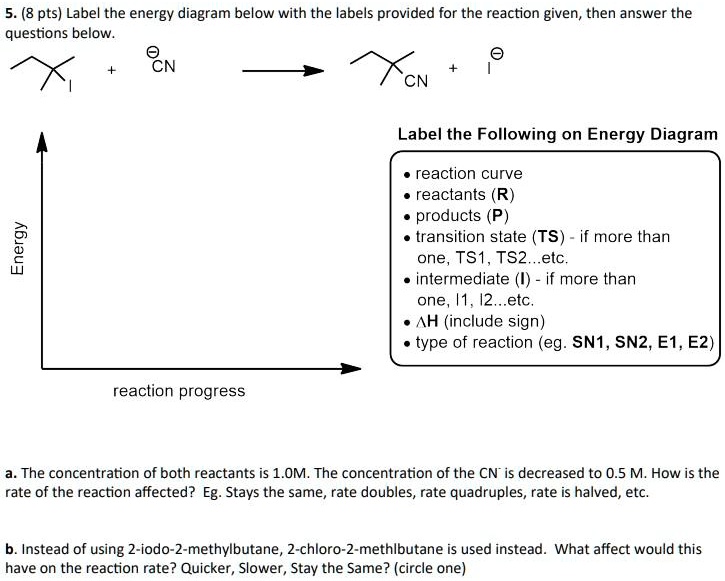
40 label the following multi-step reaction energy diagram.
PDF Reactions of Alkenes and Alkynes - Bloomsburg Area School ... step in a multistep reaction. EXAMPLE 5.1 Draw an energy diagram for a two-step exothermic reaction in which the second step is rate determining. STRATEGY A two-step reaction involves the formation of an intermedi-ate. In order for the reaction to be exothermic, the products must be lower in energy than the reactants. In order for the DOC 1 - bohr.winthrop.edu For each reaction: 1) Draw in all non-bonding electrons in the reactants and products 2) Label each reactant as either nucleophile or electrophile 3) Draw the curved arrows needed to show each reaction mechanism. b. Draw a free energy diagram for the reaction of ethanol with HBr to give bromoethane.
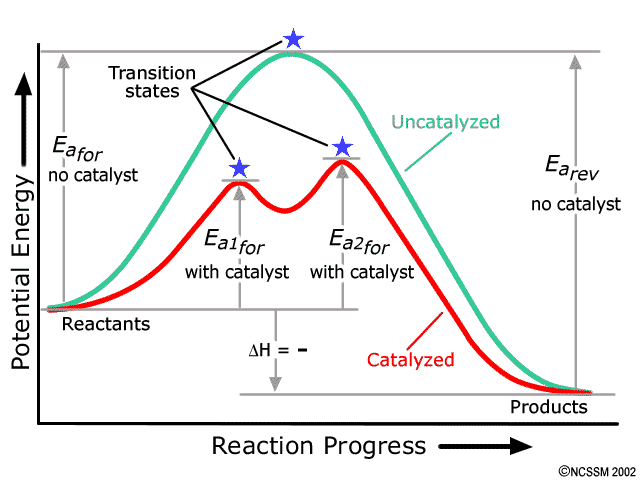
12.7 Catalysis - Chemistry - opentextbc.ca This potential energy diagram shows the effect of a catalyst on the activation energy. The catalyst provides a different reaction path with a lower activation energy. As shown, the catalyzed pathway involves a two-step mechanism (note the presence of two transition states) and an intermediate species (represented by the valley between the two ...

Label the following multi-step reaction energy diagram.
Energy Diagram for a Two-Step Reaction Mechanism by Ashima ... Energy Diagram for a Two-Step Reaction Mechanism Complete Energy Diagram for Two-Step Reaction A Two-Step Reaction Mechanism The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and PDF Creating Catalytic Connections with odels Teacher Key • Accomplish catalysis without being consumed in the reaction. • Catalyzes a specific chemical reaction. The Enzyme in Action Kit© allows you to explore how enzymatic reactions occur. Catabolism Model pieces needed 1. The gray foam piece is a model of an enzyme. Place it with the A label facing up. Assemble the two green pieces (B 1 and B 2 Diagram of a wave worksheet answers. - tiertaxi-renner.de Properties Of Waves Lab Worksheet Answers. Chemistry energy worksheet answers. c. Observe that the compressions are closer. _____22. Diagram A Diagram B Diagram C Diagram D Diagram E 2) Light waves refract when passing across the boundary between two media because a. Grade 7 McGraw Hill Glencoe - Answer Keys. 8 cm when an object is placed 32.
Label the following multi-step reaction energy diagram.. play.kahoot.itKahoot! You need to enable JavaScript to run this app. Kahoot! You need to enable JavaScript to run this app. PDF Rates, Temperature and Potential Energy Diagrams Worksheet Draw a potential energy (E p ) diagram for a reaction in which ∆H = 80 kJ/mol and E a = +28kJ/mol. Label the axes, activation energy, ∆H, site of the activated complex, reactants and products. 3. Using the potential energy diagrams for an endothermic and exothermic reaction shown, choose the letter that best fits each statement. Welcome to CK-12 Foundation | CK-12 Foundation Draw the potential energy diagram for the following multi-step reaction . Properly label the diagram. Solution: Rate of Reaction is Determined by Slowest Step In a series of reactions that make up a multi-step reaction, each individual reaction step has its own reaction rate that is determined by the factors that have been discussed in this ... DOC 1 - Winthrop University Draw a free energy diagram for the acid-catalyzed hydration of 2-butene (the last step is very fast.) Label all reactants, products and intermediates using structures. 3. Reactions. a. Draw the major product or products of each of reaction in the boxes provided. ... Be explicit when showing multi-step reactions. 4. Mechanisms.
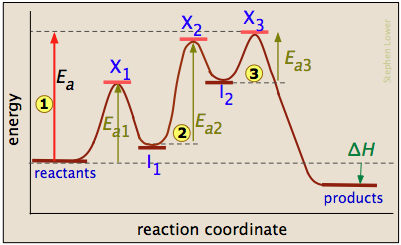
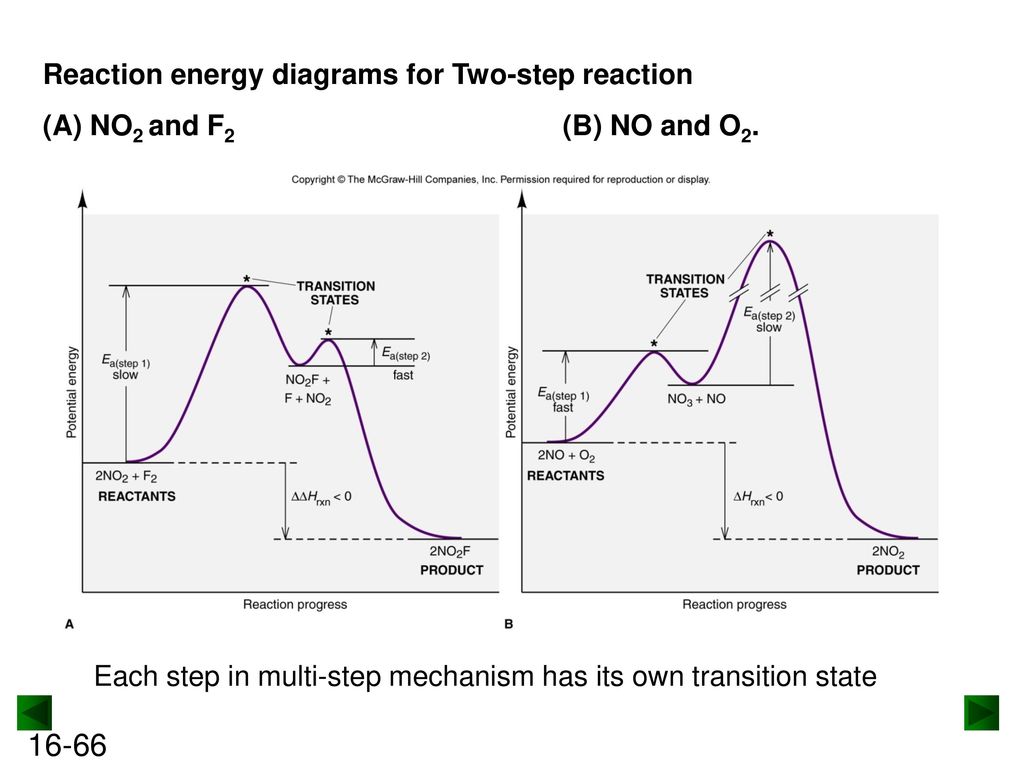
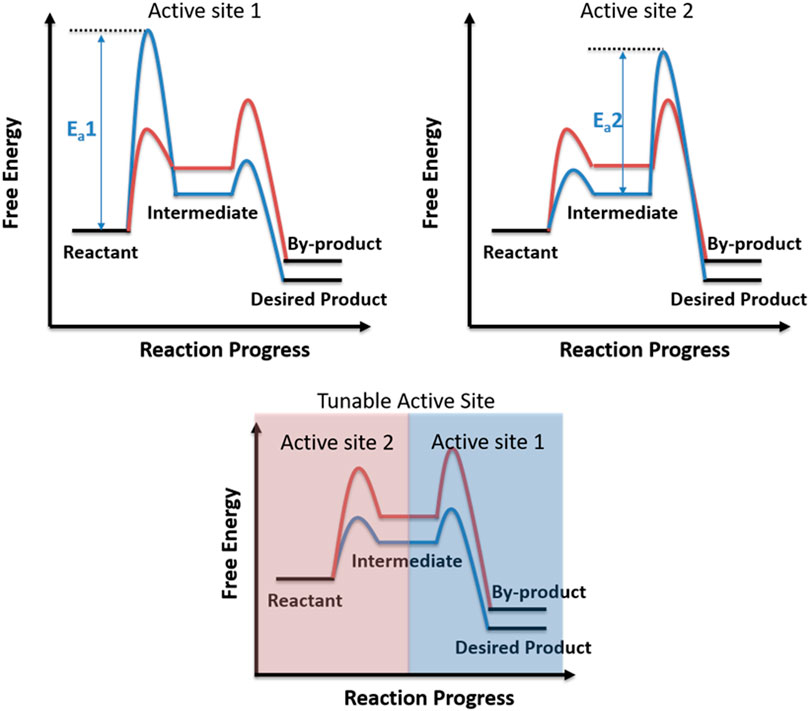
Non-ionic Chemical Reactions - Michigan State University Reaction 7 shows a similar rearrangement of a sulfur ylide to a cyclic sulfide. The [2,3]-Wittig rearrangement is yet another example. 4. Ene Reactions. The joining of a double or triple bond to an alkene reactant having a transferable allylic hydrogen is called an ene reaction. The reverse process is called a retro ene reaction. In the bonding ... 12.6 Reaction Mechanisms - Chemistry Because a reaction cannot proceed faster than its slowest step, this step will limit the rate at which the overall reaction occurs. The slowest step is therefore called the rate-limiting step (or rate-determining step) of the reaction Figure 2. Figure 2. A cattle chute is a nonchemical example of a rate-determining step. 3.03 lab_ dichotomous key answers. Diagram the feedback loop(s) involved. 03 lab_ dichotomous key answers A dichotomous key is a tool that allows the user to determine the identity of an item based on physical traits. pl Enzyme has active site (exposed R groups) where reaction occurs c. c 3. In this project, you will be creating a dichotomous key. Shares: 2. Energy Diagrams: Describing Chemical Reactions Draw an energy diagram for a two-step reaction that is exothermic overall, and consists of a fast but endothermic first step, and a slow but exothermic second step. Indicate DGrxn, as well as DG1* and DG2* for the first and second activation energies, respectively. Label the positions corresponding to the transition states with an asterisk.
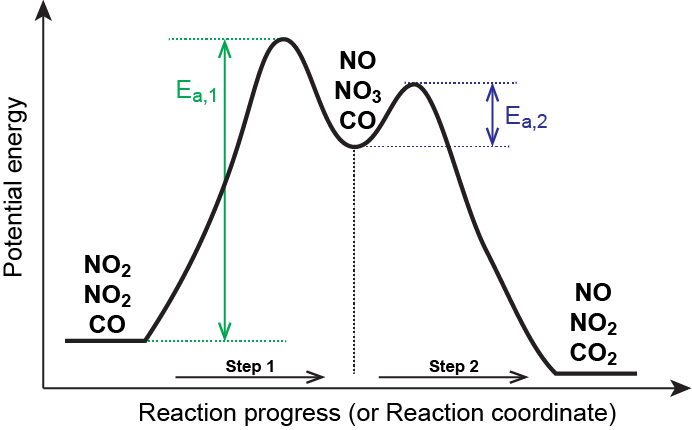
Mechanisms and Potential Energy Diagrams | Chemistry for ... A potential energy diagram for a two-step reaction is shown and labeled. Practice View the section on two-step reactions at the site below and then do the self-test (both buttons are at the top of the slide). Don't worry about - just consider it an indication of activation energy as is in the diagram above. Energy Diagram Module Series- Part Three: Intermediates ... Part 1 Part 2 Sometimes reactions are more complex than simply a transition state (Graph 3), which would represent a single step in the reaction mechanism. You will soon see most reactions proceed in a multistep fashion. In this case, reaction mechanisms often form lower energy and sometimes isolatable intermediates. PDF Rates, Temperature and Potential Energy Diagrams Worksheet a) Draw a potential energy diagram for this reversible reaction. Your starting value for the reactants might be different that is ok, as long as you show the proper E a and ∆H values. b) Calculate the enthalpy change (∆H) for each reaction. i) ΔH forward = 137 kJ/mol i) ΔH reverse = 137 kJ/mol 6. This review was not prepared by your professor or school ... UCONN - GENERAL CHEMISTRY - PROF. CADY UCONN CHEM 1128Q CADY EXAM 1 REVIEW* PRACTICE: Label the multi-step reaction energy diagram below using the letters corresponding to the labels on the left. There are more labels than needed; each label can be used only once.
Energy Diagrams of Two Step Reactions - YouTube Watch Complete videos @ Organic Chemistry 1
PDF 5.12 Spring 2003 Review Session: Exam #2 reaction. Use fishhook arrows! • Draw and completely label a reaction-energy diagram. • Deter min e th ra te-d ter ing sp of a ulti- ep re act ion-energy d gram. • Differentiate between transition states and intermediates. • Differentiate between kinetic and thermodynamic control. • Use the Hammond postulate to predict whether a
ENERGY PROFILES FOR SIMPLE REACTIONS - chemguide This diagram shows that, overall, the reaction is exothermic. The products have a lower energy than the reactants, and so energy is released when the reaction happens. It also shows that the molecules have to possess enough energy (called activation energy) to get the reactants over what we think of as the "activation energy barrier".
MCB: Exam 4 Sapling Flashcards - Quizlet Label the diagram with the appropriate terms to describe glycogen synthase regulation. ... The multi‑step activation of a fatty acid involves the hydrolysis of the equivalent of two molecules of ATP. The overall free energy change for the reaction includes the free energy change for the hydrolysis of ATP and the subsequent hydrolysis of PPi.
PDF 5.111 F14 Final Exam Solutions - MIT OpenCourseWare 406 M-1 min-1 and the rate constant for the reverse reaction is 244 M-1 min-1. The activation energy for the forward reaction is 26.2 kJ mol-1 and that for the reverse reaction is 42.4 kJ mol-1. (a) (5 points) On the axes below, draw a reaction coordinate diagram for this reaction, showing the
courseworkhero.co.ukCoursework Hero - We provide solutions to students We provide solutions to students. Please Use Our Service If You’re: Wishing for a unique insight into a subject matter for your subsequent individual research;
Chapter 14 B Flashcards - Quizlet The reaction is exothermic because the energy of the products is less than the energy of the reactants Intermediates are dips (2) in this case, and they are formed at different rates Consider the following multi-step reaction:
OneClass: Label the following multi-step reaction energy ... 11. Label numbers 1-4 on the energy diagram: Label the activation energies on the diagram. Is the overall reaction endothermic or exothermic? 3. What is the reactant for the rate determining step? Reaction Path 27. Calculate the pH of a 0.0sS M solution of CHCNa (K,(C)-18x10)
Energy Profiles (Energy Diagrams) Chemistry Tutorial From our energy profile diagram we see that 192.4 kJ mol -1 of energy was absorbed by the reactant molecules, but only 100 kJ mol -1 was released as the activated complex broke apart to make the product molecules. Overall, the system absorbed a net amount of energy of 192.4 - 100 = 92.4 kJ mol -1 .
PDF Organic Chemistry 32-235 Practice Questions for Exam #2 ONE 2) Draw an energy diagram (using the ∆H° for each step to set appropriate energy levels) for the two propagation steps, and decide which step is more likely the rate-determining step? (label this step as rds) see the textbook for chlorination of methane and apply it to this case. energy reaction coordinate (progress) CH4 + Br2 CH3Br + HBr
Solved Label the following multi-step reaction energy ... Label the following multi-step reaction diagram. Show transcribed image text. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 99% (114 ratings) Transcribed image text: Label the following multi-step reaction energy diagram.
Energy Diagram Catalyzed Vs Uncatalyzed Reaction Label the energy diagram and answer the question that follows% (1). The decomposition of hydrogen peroxide is exothermic. The reaction is catalyzed by iodide ion. The equation for the uncatalyzed reaction is. 2 H 2 O 2 (l) 2 H 2 O (l) + O 2 (g) Sketch a possible graph for this reaction, first without a catalyst and then with a catalyst.
Solved Label the following multi-step reaction energy ... Question: Label the following multi-step reaction energy diagram AHn> 0 Reaction Intermediate Ealstep 2) Products Ea (step 1) Reactants Reaction progress This problem has been solved! See the answer Show transcribed image text Expert Answer 100% (4 ratings)
Diagram of a wave worksheet answers. - tiertaxi-renner.de Properties Of Waves Lab Worksheet Answers. Chemistry energy worksheet answers. c. Observe that the compressions are closer. _____22. Diagram A Diagram B Diagram C Diagram D Diagram E 2) Light waves refract when passing across the boundary between two media because a. Grade 7 McGraw Hill Glencoe - Answer Keys. 8 cm when an object is placed 32.
PDF Creating Catalytic Connections with odels Teacher Key • Accomplish catalysis without being consumed in the reaction. • Catalyzes a specific chemical reaction. The Enzyme in Action Kit© allows you to explore how enzymatic reactions occur. Catabolism Model pieces needed 1. The gray foam piece is a model of an enzyme. Place it with the A label facing up. Assemble the two green pieces (B 1 and B 2
Energy Diagram for a Two-Step Reaction Mechanism by Ashima ... Energy Diagram for a Two-Step Reaction Mechanism Complete Energy Diagram for Two-Step Reaction A Two-Step Reaction Mechanism The transition states are located at energy maxima. The reactive intermediate B+ is located at an energy minimum. Each step has its own delta H and


















0 Response to "40 label the following multi-step reaction energy diagram."
Post a Comment