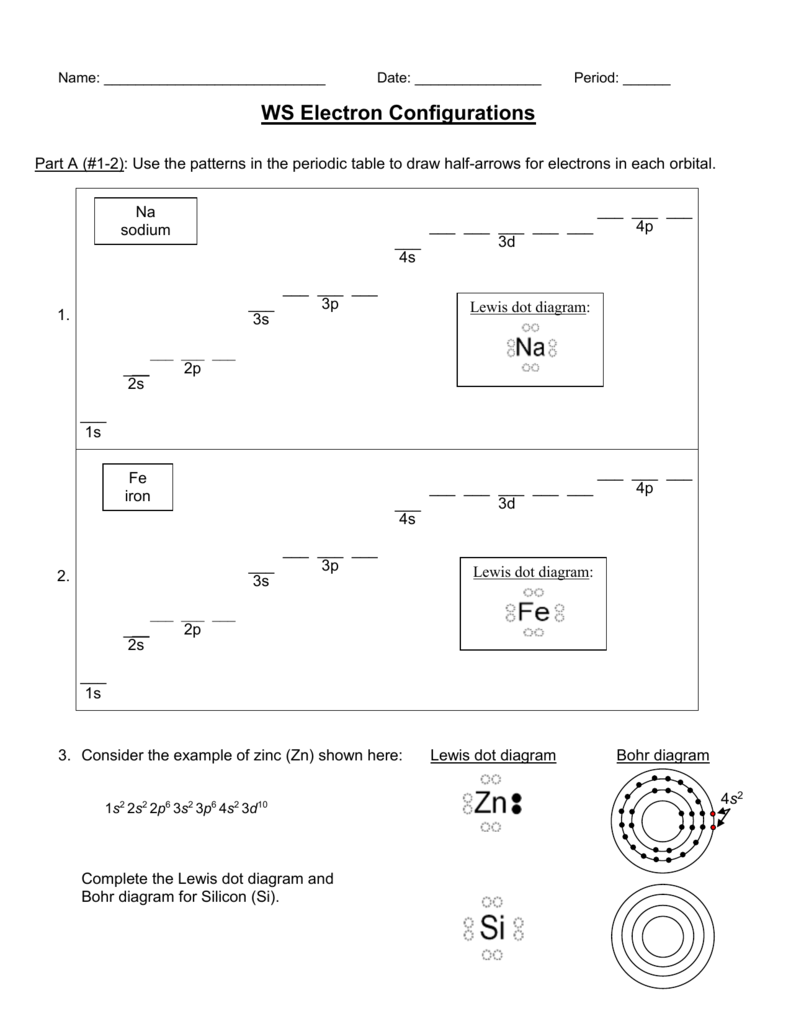
41 orbital diagram for zinc
Orbital Diagram of Zinc (Zn), Electron Configuration, and ... Orbital Diagram, electron configuration, and the noble gas notation for a zinc (Zn) atom. The orbital diagram for the ground state of Zinc and its ... The orbital diagram for the ground state of Zinc and its magnetic nature has to be written. Concept introduction: Pauli Exclusion Principle An orbital having a most two electrons and in this two electrons have opposite spins. Each orbital having no more than two electrons and similar spin is not allowed. Aufbau principle
Choose The Valence Orbital Diagram That Represents The ... Choose the valence orbital diagram that represents the ground state of zn. Choose the valence orbital diagram that represents the ground state of se2⁻. One electron can jump from a lower to a higher energy orbital, thus creating unpaired electrons. A partial orbital diagram shows only the highest energy sublevels being filled.

Orbital diagram for zinc
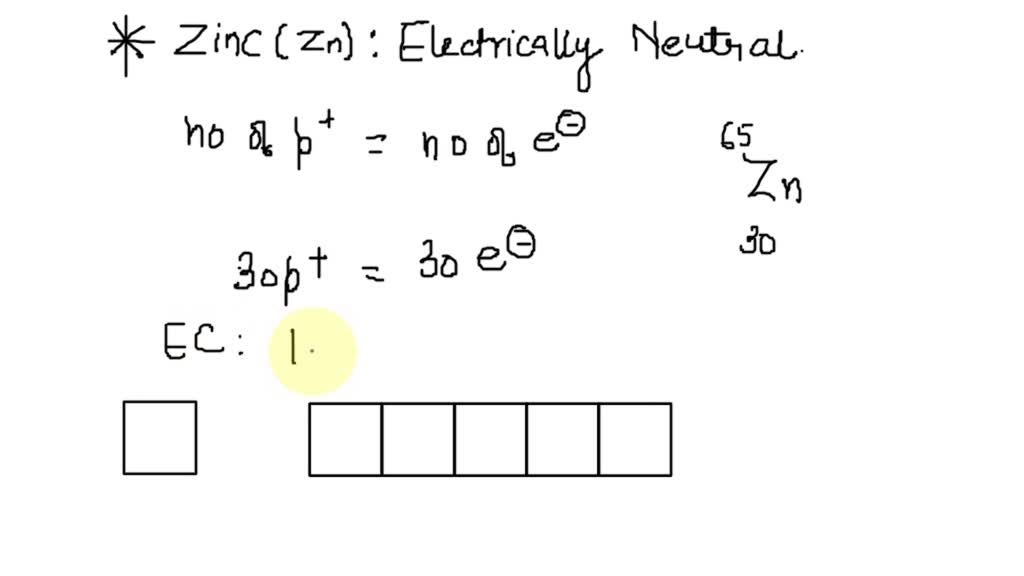
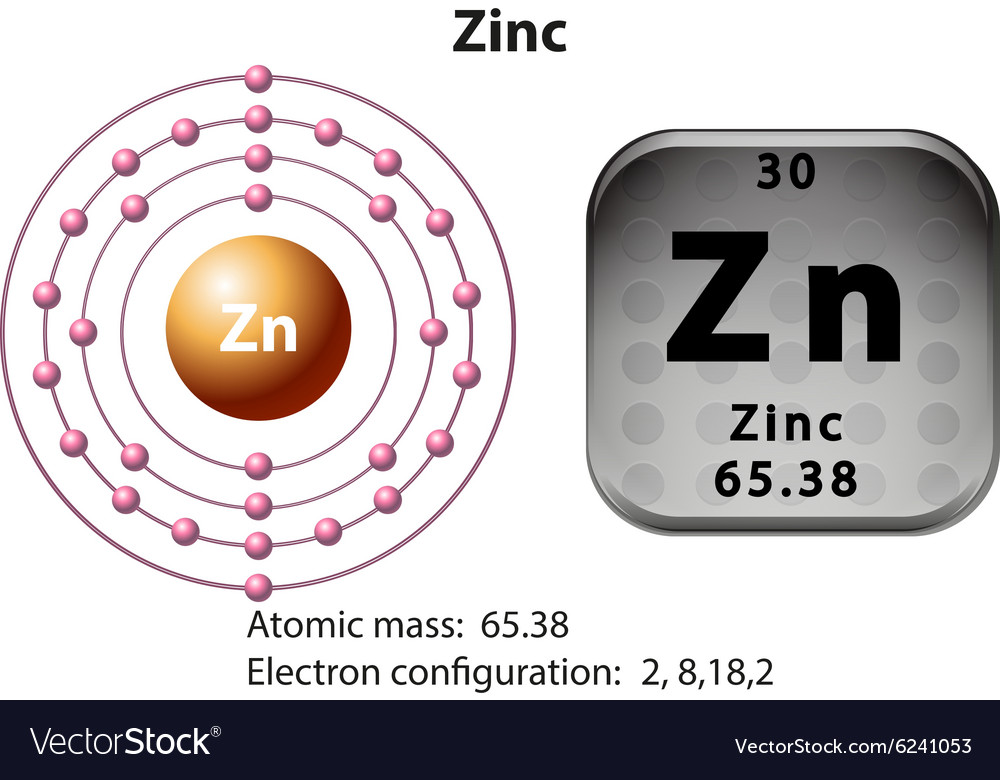
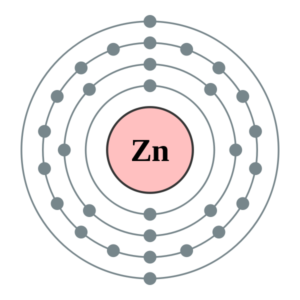
Calcium Orbital Filling Diagram - schematron.org For example, the 5s orbital is of lower energy than the 4d orbital (see Figure 3 for a complete pattern of orbital levels). Ca electron occupancy The orbital notation of calcium (Ca) is 1s2 2s2 2p6 3s2 3p6 4s2 Or short-hand: [Ar] 4s2. The orbital filling diagram of lithium. The electron configuration of lithium is 1s²2s¹. What is the electron dot diagram for zinc class 12 ... Hint: Electron dot diagram also known as Lewis diagram is a way to represent the number of electrons present in the valence shell of an element.In the diagram, the number of dots on the symbol of the element represent its valence electrons. Complete answer: To find the electron dot diagram for zinc, we need to write its electronic configuration according to Aufbau's Principle. Zinc, atomic structure - Stock Image - C018/3711 - Science ... Zinc (Zn). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of zinc-65 (atomic number: 30), an isotope of this element. The nucleus consists of 30 protons (red) and 35 neutrons (orange). 30 electrons (white) successively occupy available electron shells (rings).
Orbital diagram for zinc. What is the orbital notation for zinc? - Answers What is the orbital notation for zinc? - Answers 1s with two arrows, 2s with 2 arrows, 2p with 6 arrows, 3s with 2 arrows, 3p with 6 arrows, 4s with 2 arrows, 3d with 10 arrow; Remember that... Orbital Diagram of Zinc (Zn), Electron Configuration, and ... Jun 6, 2018 - Orbital Diagram, electron configuration, and the noble gas notation for a zinc (Zn) atom. PDF Orbital diagram for zinc - static.s123-cdn-static.com Orbital diagram for zinc Continue Orbital diagram for zinc Look at the board. The field on the left has all the information you need to know about one element. This will tell you the weight of one atom, how many pieces are inside, and where it should be placed on the periodic table. Now we work with the fourth period / row in the table of elements. Orbital Diagram For Selenium - schematron.org In writing an. Answer to orbital diagram for selenium home / study / science / chemistry / chemistry questions and answers / Orbital Diagram For Selenium. here is the electronic configuration. Z=34 so 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4.Selenium (Se) has an atomic mass of Find out about its chemical and physical properties, states, energy ...
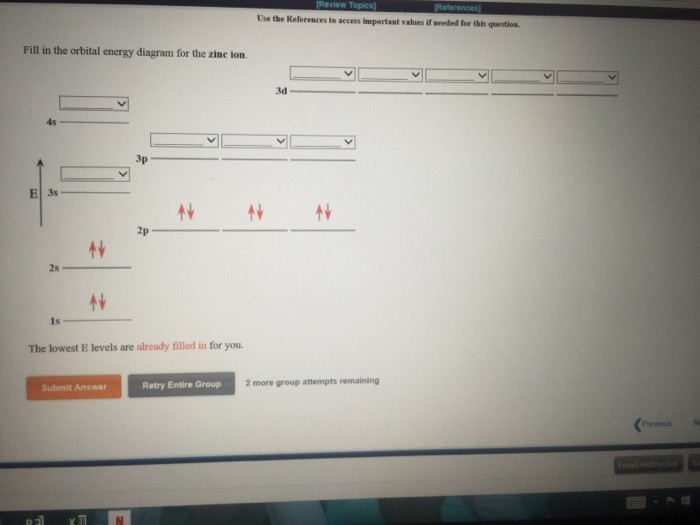
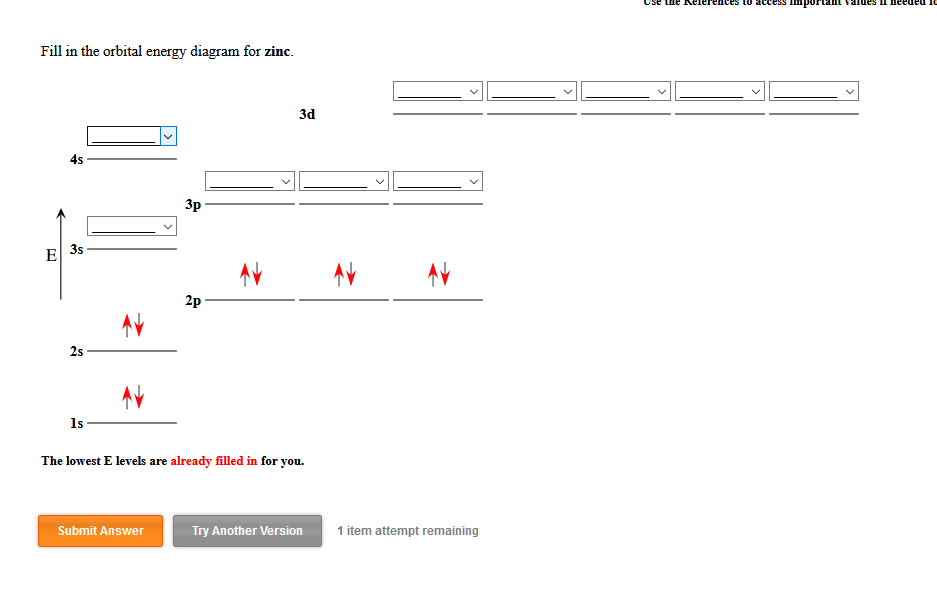
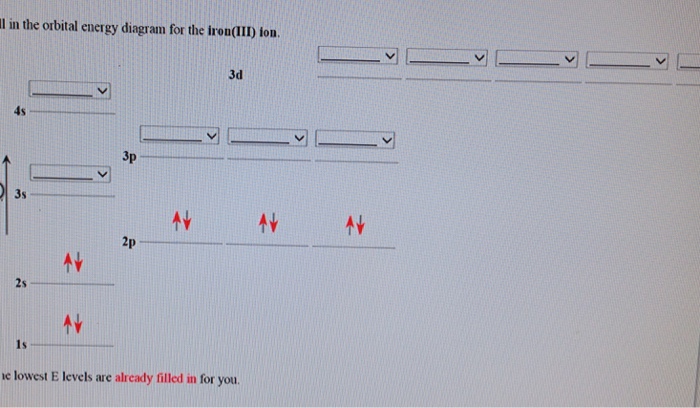
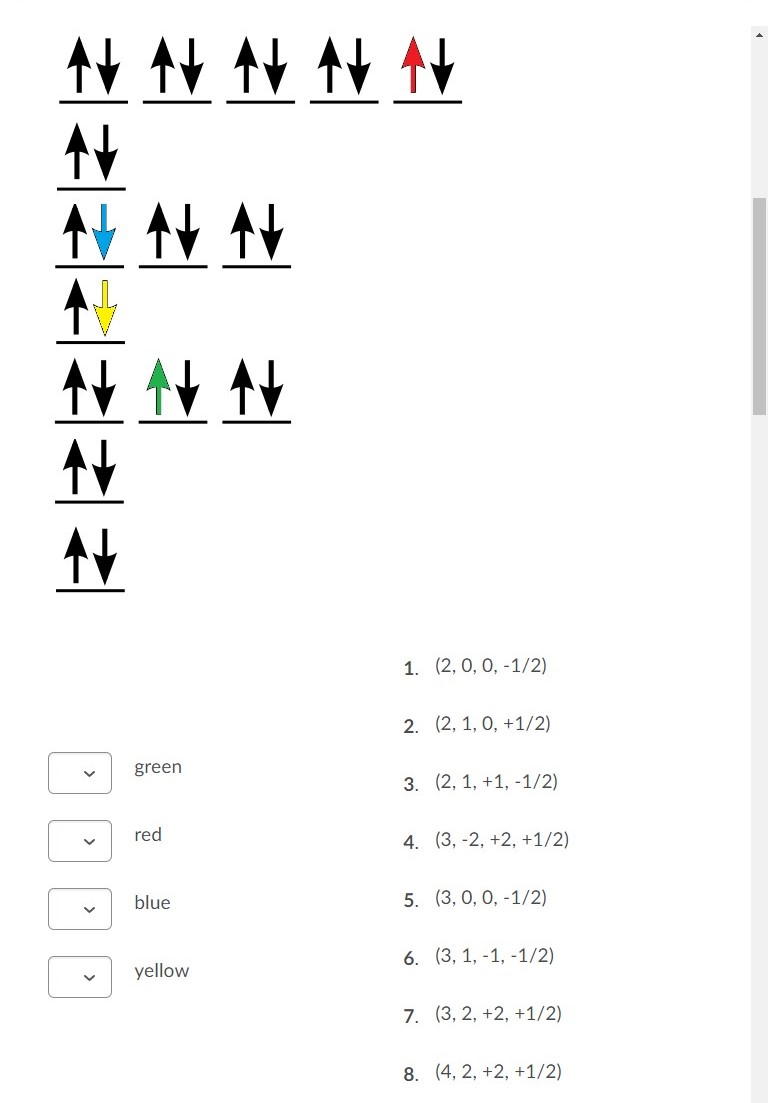
Solved Fill in the orbital energy diagram for the zinc ion ... Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (4 ratings) Transcribed image text: Fill in the orbital energy diagram for the zinc ion The lowest E levels are already filled in for you. What is the electron configuration for Zn2+? | Socratic Zinc is a d-block element, also known as a transition element. For the d-block elements, the outermost s-sublevel has higher energy than the d-sublevel, which is contrary to what the Aufbau diagram indicates. When d-block elements lose electrons, they lose the highest energy s electrons first, which in the case of zinc are the two 4s electrons. PDF Electron Configurations and Orbital Diagrams key 1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals? How many unpaired electrons are in a zinc atom? Solution. Zero. All electrons are paired in a neutral zinc atom in its ground state. The electron configuration of a neutral zinc atom in its ground state is 1s 22s 22p 63s 23p 63d 104s 2. The fact that the electron configuration shows that all sublevels are full, indicates that there are no unpaired electrons.
Solved Fill in the orbital energy diagram for the zinc ion ... Science. Chemistry. Chemistry questions and answers. Fill in the orbital energy diagram for the zinc ion 3d 45 3p E 35 AV NV 2p NV 25 AV ls. PDF Orbital diagram for zinc ion - static.s123-cdn-static.com Orbital diagram for zinc ion Continue Orbital diagram for zinc ion The purpose of the introduction of quantum numbers is to show that similarities in the electron control or electron configuration lead to the similarities and differences in the properties of elements. What is the orbital diagram for zinc? - AnswersToAll Zinc is a d-Block element and belongs to Transition Metal. Valence shell contains 2 electrons that is 4s2 that means that zinc can lose the two electrons located in the 4s-orbital to be become the Zn2+ cation. So, the valency of Zinc is 2. Mo3+ Orbital Diagram - Wiring Diagrams Mo3+ Orbital Diagram. Compact version of orbital energy diagram with each orbital represented . Mo2+, Mo3+, Mo4+ and Mo5+ are all known in aqueous solution. That's why Mo3+ has 39 electrons instead of 42 in its ground state electron The normal electron configuration of zinc is [Ar] 3d 10 4s 2, with 2 4s orbital.
Write an orbital diagram for the ground state of the zinc ... What is the ground state electron configuration and orbital diagram of Os(76)? Is this paramagnetic or diamagnetic? (b) The following is an excited state configuration of a neutral atom: 1s22s22p53s1 3p44s2 5s26s15p5 Identify the element and write its ground state electronic configuration.
Answered: Fill in the orbital energy diagram for… | bartleby Fill in the orbital energy diagram for zinc. 3d 4s Зр E 3s 2p 2s Common Greek (upper) Greek (lower) Arrows Other 1s xº x, x Aa (s) (aq) The lowest E levels are already filled in for you. Undo Redo Clear Help Submit Answer Retry Entire Group 9 more group attempts remaining Question
Zinc atomic orbital diagram example. - YouTube Zinc. Atomic Orbital. Electron orientation
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium (Se) 35: Orbital diagram of Bromine (Br) 36: Orbital diagram of Krypton (Kr) 37: Orbital diagram of Rubidium (Rb) 38: Orbital diagram of Strontium (Sr) 39:
QUIZ 10-GENERAL CHEM I Flashcards | Quizlet Choose the valence orbital diagram that represents the ground state of Zn. Give the ground state electron configuration for Se. [Ar]4s23d104p4. Give the ground state electron configuration for I. [Kr]5s24d105p5. Give the ground state electron configuration for Pb. [Xe]6s2 4f14 5d10 6p2.
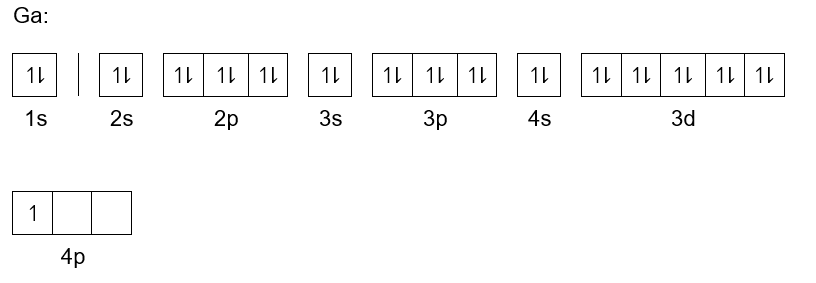
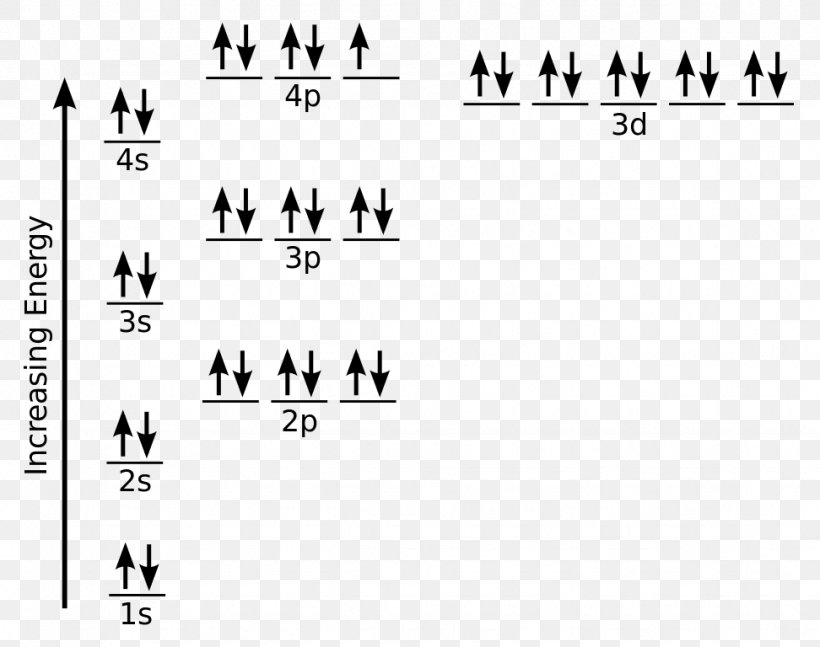
Arrangements of electrons in the orbitals of an atom is ... Zinc has an electron configuration of [Ar]4s 2 3d 10. At gallium we begin filling the 4p sublevel and continue to krypton. Rubidium fills the 5s, yttrium the 4d and indium the 5p. Cesium fills the 6s and lanthanum bigins the first available f sublevel, the 4f. The f sublevel is filled from lanthanum through ytterbium.
zinc orbital diagram - Pastor Choolwe Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of zinc-65 (atomic number: 30), an isotope of this element. The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. Zinc (Zn). So the only possible stable configuration would be 4s2 3d3.
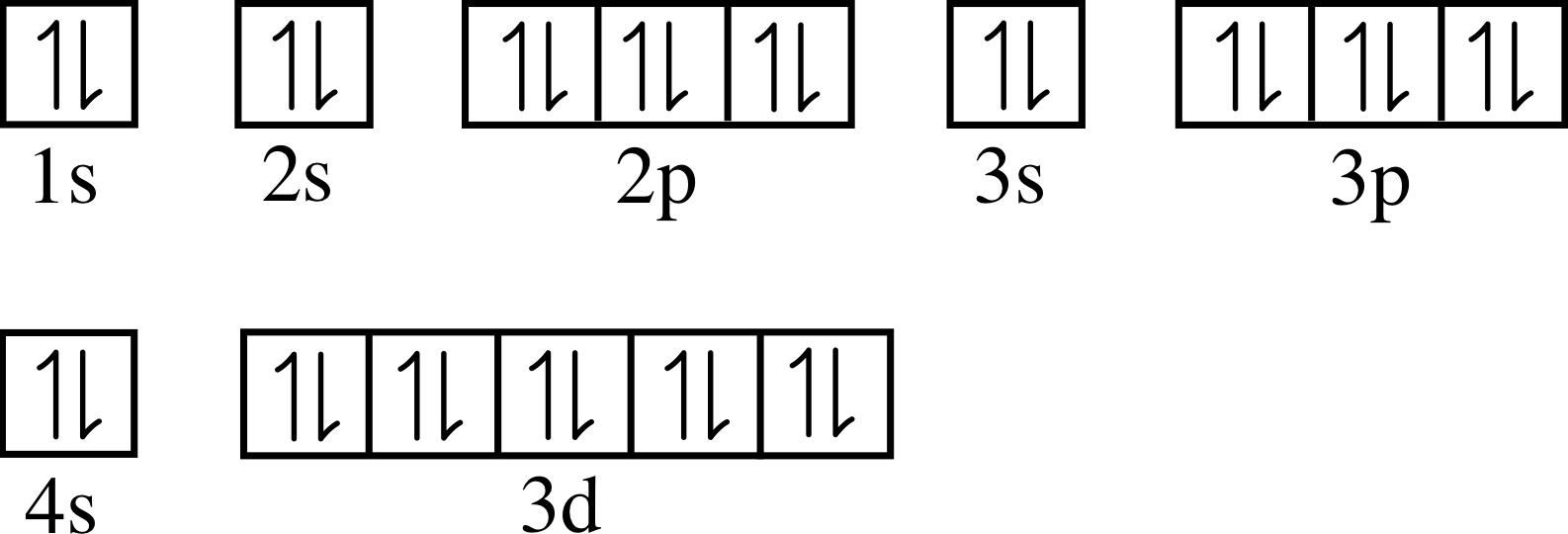
WebElements Periodic Table » Zinc » properties of free atoms Binary compounds. Compound properties. Element reactions. Zinc atoms have 30 electrons and the shell structure is 2.8.18.2. The ground state electron configuration of ground state gaseous neutral zinc is [ Ar ]. 3d10. 4s2 and the term symbol is 1S0. Schematic electronic configuration of zinc. The Kossel shell structure of zinc.
Copper(Cu) electron configuration and orbital diagram According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction. The 1s orbital is now filled with two electrons. Then the next two electrons will enter the 2s orbital just like the 1s orbital.
Electron configuration for Zinc (element 30). Orbital diagram Zn (Zinc) is an element with position number 30 in the periodic table. Located in the IV period. Melting point: 419.6 ℃. Density: 7.14 g/cm 3 . Electronic configuration of the Zinc atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10.
Zinc(Zn) electron configuration and orbital diagram Atomic Orbital Diagram for Zinc (Zn) Zinc ion (Zn 2+) electron configuration Ground state electron configuration of zinc (Zn) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2. The electron configuration shows that the last shell of zinc has two electrons and the d-orbital has a total of ten electrons. In this case, the valence electrons of zinc (Zn) are two.
Zinc, atomic structure - Stock Image - C018/3711 - Science ... Zinc (Zn). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of zinc-65 (atomic number: 30), an isotope of this element. The nucleus consists of 30 protons (red) and 35 neutrons (orange). 30 electrons (white) successively occupy available electron shells (rings).
What is the electron dot diagram for zinc class 12 ... Hint: Electron dot diagram also known as Lewis diagram is a way to represent the number of electrons present in the valence shell of an element.In the diagram, the number of dots on the symbol of the element represent its valence electrons. Complete answer: To find the electron dot diagram for zinc, we need to write its electronic configuration according to Aufbau's Principle.
Calcium Orbital Filling Diagram - schematron.org For example, the 5s orbital is of lower energy than the 4d orbital (see Figure 3 for a complete pattern of orbital levels). Ca electron occupancy The orbital notation of calcium (Ca) is 1s2 2s2 2p6 3s2 3p6 4s2 Or short-hand: [Ar] 4s2. The orbital filling diagram of lithium. The electron configuration of lithium is 1s²2s¹.


























0 Response to "41 orbital diagram for zinc"
Post a Comment