40 molecular orbital diagram for ne2 2+
Solved Draw The Valence Bond Lewis Structure of Ne2^+2 ... This problem has been solved! Draw The Valence Bond Lewis Structure of Ne2^+2. Draw Molecular Orbital Diagram using Shorthand Notation. What is the bond order, number of sigma bonds, number of pi bonds? Is it paramagnetic? Who are the experts? Experts are tested by Chegg as specialists in their subject area. electronic configuration - Molecular orbital (MO) diagram ... The short answer is: we could not tell it using the primitive molecular orbital theory introduced in the general chemistry courses. In exact same way we could not tell why $\mathrm{\sigma_{2p_{z}}}$ MO becomes lower in energy than $\mathrm{\sigma_{2p_{z}}}$ MO to the left of $\ce{N2}$ and not to the left of, say, $\ce{C2}$.
with the help of molecular orbital theory show that ne2 ... According to Molecular Orbital theory, only those molecule can exists which have net positive bond order while the molecules with negative or zero bond order will not exists. In case of Ne 2 molecule, since, Ne atom has 10 electrons so total electrons are 20 the configuration is given as:

Molecular orbital diagram for ne2 2+
Molecular Orbital Diagram Ne2 - schematron.org For Ne 2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 schematron.org each MO an appropriate label. Determine the electron configuration and bond order for each, and rank the three species in order of increasing bond order% (1). F2 Molecular Orbital Diagram - 17 images - what is the ... Here are a number of highest rated F2 Molecular Orbital Diagram pictures upon internet. We identified it from well-behaved source. Its submitted by processing in the best field. We take this kind of F2 Molecular Orbital Diagram graphic could possibly be the most trending subject in the manner of we share it in google lead or facebook. Molecular Orbital Theory | Chemistry for Majors A molecular orbital can hold two electrons, so both electrons in the H 2 molecule are in the [latex]\sigma[/latex] 1s bonding orbital; the electron configuration is [latex]{\left({\sigma}_{1s}\right)}^{2}.[/latex] We represent this configuration by a molecular orbital energy diagram (Figure 10) in which a single upward arrow indicates one ...
Molecular orbital diagram for ne2 2+. Molecular Orbital Theory - Purdue University Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... Molecular Orbital Diagram For Ne2 - schematron.org Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways.Draw the molecular orbital diagram for Ne 2 + and determine if the bond between the two atoms will be stable. 7.7 Molecular Orbital Theory - Chemistry Fundamentals A molecular orbital can hold two electrons, so both electrons in the H 2 molecule are in the [latex]\sigma[/latex] 1s bonding orbital; the electron configuration is [latex]{\left({\sigma}_{1s}\right)}^{2}.[/latex] We represent this configuration by a molecular orbital energy diagram (Figure 7.7.10) in which a single upward arrow indicates one ... › ncert-solutions-for-class-11NCERT Solutions for Class 11 Chemistry Chapter 4 - LearnCBSE.in Question 35. Use molecular orbital theory to explain why the Be 2 molecule does not exist. Answer: Question 36. Compare the relative stability of the following species and indicate their magnetic properties: O 2, O 2, O 2 – (Superoxide),O 2 2-(peroxide) Answer: O 2 — Bond order = 2, paramagnetic O 2 + — Bond order = 2.5, paramagnetic
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ... Molecular Orbital Diagram of O2, F2, and Ne2 Molecules ... 0:21 Molecular Orbital Diagram of Oxygen Molecule3:30 Molecular Orbital Diagram of Florine Molecule5:25 Molecular Orbital Diagram of Neon MoleculeSo as we d... Draw the molecular orbital diagram of N 2 . Also find its ... Molecular orbital diagram of N 2 BO = [Nb-Na] = [10-4] = 3 Since all the electrons in nitrogen are paired, it is diamagnetic molecule. Answered by | 13th Jun, 2016, 04:45: PM. Concept Videos. Molecular Orbital Theory - Part 1. Solved Draw the molecular orbital (MO) electron diagram ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 90% (10 ratings) Transcribed image text: Draw the molecular orbital (MO) electron diagram for the Ne2 molecular ion. Be sure your diagram contains all of the electrons in the ion, including any core electrons.
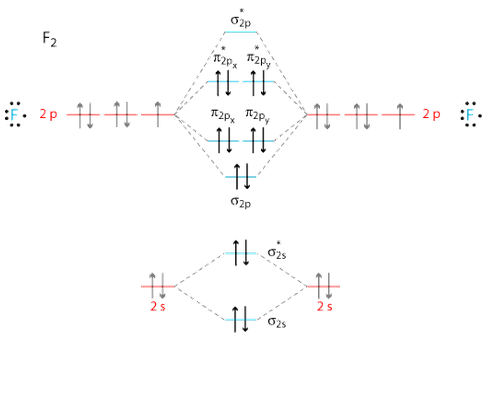
Choose the correct molecular orbital diagram for F 2 ... Choose the correct molecular orbital diagram for F 2 . (NOT both!) a) Label all of the molecular orbitals (on one diagram only). b) Show the electrons in the molecular orbital diagram for F 2 . c) Indicate whether the molecular ion is paramagnetic or diamagnetic? d) Calculate the bond order. e) What... 38 molecular orbital diagram for ne2 2+ - Wiring Diagrams ... Molecular orbital diagram for ne2 2+. To form the 2+ ion, the uppermost electrons in the sigma* 2p orbital are removed, making it isoelectronic with F2, so it has a bond order of 1 and should be ... PDF MO Diagrams for Diatomic Molecules The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine › 42397273 › Chemistry_the_centralChemistry the central science 14th edition - Academia.edu Academia.edu is a platform for academics to share research papers.
Recitation Week 10 (test 3 - Recitation 2) - GitHub Pages A) O2^2-B) Ne2^2+ C) O2^2+ D) F2^2+ E) None of the above are paramagnetic; 3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. A) B2^2+ B) B2^2-C) N2^2+ D) C2^2-E) B2; 4) Draw the molecular orbital diagram shown to determine which of the following is most stable.
Energy level diagram for Molecular orbitals - Chemical ... Energy level diagram for Molecular orbitals. ... If N b = Na,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of electron in the bonding molecular orbitals. 2) Stability of molecules in terms of bond order.
MO Diagram for N2+ (Molecular Orbital) - YouTube There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start...
What is the molecular orbital diagram for B_2? | Socratic Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the 1s orbitals, because they do not contain the valence electrons. Each boron atom has one 2s and three 2p valence orbitals. The 2s orbitals will overlap to form 2sσ and 2sσ ...
PDF Simple Molecular Orbital Theory - University of California ... LCAO MO Energy Diagram for H2 Energy H-H ∆E1 ∆E2 • ∆E2> ∆E1, so the antibonding orbital is always more anti-bonding than the bonding orbital is bonding H2molecule: two 1s atomic orbitals combine to make one bonding and one antibonding molecular orbital. Ha Hb
Molecular Orbital Diagram Ne2 - Diagram Niche Ideas Molecular orbital diagram ne2.If ne 2 did form, it would be diamagnetic. Once you have the molecular orbitals and their energy ordering the ground state configuration is found by applying the pauli principle, the what is the molecular orbital diagram for the diatomic neon molecule, ne2?
Molecular Orbital Diagram For Ne2 According to Molecular Orbital theory, only those molecule can exists which have net positive bond order while the molecules with negative or. Answer to For Ne2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 anion. © Prof Adam J Bridgeman | close window.
Draw molecular orbital diagram of O2 or N2 with magnetic ... As it can be seen from the MOT of O 2. . , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [N b. . −N a. . ]/2=[10−6]/2=2. Therefore there is a double bond present as O=O.
Draw a molecular orbital diagram of ${N_2}$ or ${O_2 ... Now, let us draw the molecular orbital diagram of ${N_2}$ . Now, first let us understand what magnetic behavior and bond order means. - Magnetic behavior: As we know the electron has an electron magnetic dipole moment, which is generally generated by the electron's spin property, which induces an electric charge into motion. As we can see the ...
› 49212961 › Inorganic_Chemistry_by(PDF) Inorganic Chemistry by Miessler ~ 5th Edition | Arnab ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry.
Li2 Mo Diagram In this section, we will compare MO diagrams for diatomic molecules X-X, from Li 2 to Ne2. We will predict their bond order and see how the. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining . However, experimental and computational results for homonuclear diatomics from Li2 to N2 and certain heteronuclear ...
Chapter 11: Theories of Covalent Bonding Smartbook ... Using the attached molecular orbital energy diagram, select the correct molecular electron configuration for Ne2 2+. (Only molecular orbitals formed from valence atomic orbitals have been included.) A. (σ2s)2(σ∗2sσ2s )2(σ2p)2(π2p)2(π∗2pπ2p
Use the molecular orbital energy level diagram to show ... Use the molecular orbital energy level diagram to show that N 2 would be expected to have a triple bond, F 2 , a single bond and Ne 2 , no bond. Hard Solution Verified by Toppr Formation of N 2 molecule: Electronic Configuration, σ1s 2<σ∗1s 2<σ2s 2<σ∗2s 2<[π2p x2 =π2p x2 ]<<σ2p z2 Bond order = (N b −N a )/2=(10−4)/2=3
Molecular Orbital Theory | Chemistry for Majors A molecular orbital can hold two electrons, so both electrons in the H 2 molecule are in the [latex]\sigma[/latex] 1s bonding orbital; the electron configuration is [latex]{\left({\sigma}_{1s}\right)}^{2}.[/latex] We represent this configuration by a molecular orbital energy diagram (Figure 10) in which a single upward arrow indicates one ...
F2 Molecular Orbital Diagram - 17 images - what is the ... Here are a number of highest rated F2 Molecular Orbital Diagram pictures upon internet. We identified it from well-behaved source. Its submitted by processing in the best field. We take this kind of F2 Molecular Orbital Diagram graphic could possibly be the most trending subject in the manner of we share it in google lead or facebook.
Molecular Orbital Diagram Ne2 - schematron.org For Ne 2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 schematron.org each MO an appropriate label. Determine the electron configuration and bond order for each, and rank the three species in order of increasing bond order% (1).
0 Response to "40 molecular orbital diagram for ne2 2+"
Post a Comment