40 molecular orbital diagram for be2
Find out the bond order of H2 , H2+ , He2 , Li2 , Be2 , B2 ... In order to predict the bond order, molecular orbital diagram for H2- is to be drawn. According to MOT number of atomic orbitals combined is equal to total number of molecular orbitals formed.Electronic configuration of H is 1s1. when two hydrogen atoms come closer, then on combining two 1s orbitals,two molecular orbitals are formed among which one is bonding and other is antibonding molecular ... Molecular Orbital Diagram For Li2 - schematron.org This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be . Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation.
Be2 Molecular Orbital Diagram - schematron.org Answer to Draw an MO energy diagram and predict the bond order of Be2+ and Be2−. Do you expect these molecules to exist in the. Be2 molecule is formed by the overlap of atomic orbitals of both beryllium atoms. B2 molecule is formed by the overlap of atomic orbitals of both boron atoms.

Molecular orbital diagram for be2
Use molecular orbital theory to explain why Be2 molecule ... Use molecular orbital theory to explain why Be2 molecule does not exist. CLASSES AND TRENDING CHAPTER class 5 The Fish Tale Across the Wall Tenths and HundredthsParts and Whole Can you see the Pattern? class 6 Maps Practical Geometry Separation of SubstancesPlaying With Numbers India: Climate, Vegetation and Wildlife class 7 40 molecular orbital diagram for he2+ - Diagram For You So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = . 5 * (# of bonding electrons - # of antibonding electrons). However, when you draw the Lewis structure of B2, you get a triple bond . On the basis of molecular orbital theory, explain why `He ... Be2 Molecular Orbital Diagram Nov 11, · As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. As discussed in class it is not a bond.
Molecular orbital diagram for be2. SOLVED:Draw an MO energy diagram and predict the bond ... Let's first draw the molecular orbital energy diagram where we have sigma to us at the bottom, then sigma to a star P two P, sigma two P. P two P. Star and sigma two P star. This is what we will see for beryllium, where these two are switched in comparison to the elements that are farther to the right on the periodic table. What Is The Bond Order Of Be2− What is the bond order of B2 −?, So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = . 5 * (# of bonding electrons - # of antibonding electrons). However, when you draw the Lewis structure of B2, you get a triple bond.. Furthermore, How many valence electrons are in Be2 −?, Draw the molecular orbital (MO) electron d... | Clutch Prep Construct the molecular orbital diagram for Be2- while applying the necessary rules in filling up the orbitals. Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Recall that the bonding MOs are those without an asterisk (e.g., σ1s), while the antibonding MOs are those with an asterisk ... 8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution We draw a molecular orbital energy diagram similar to that shown in Figure 8.37.
7.7 Molecular Orbital Theory - Chemistry Fundamentals molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right. Each horizontal line represents one orbital that can hold two electrons. The molecular orbitals formed by the combination of the atomic orbitals are shown in the center. Use molecular orbital theory to explain why the Be2 ... Use molecular orbital theory to explain why the Be2 molecule does not exist. Answer The electronic configuration of Beryllium is 1s 2 2s 2. From the electronic configuration it is clear that there is no singly filled atomic orbital present in beryllium. PDF MO Diagrams for Diatomic Molecules MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine Draw the molecular orbital diagram for: (i) Be2 (ii) O2 ... Draw the molecular orbital diagram for: (i) Be2 (ii) O2 and predict bond order, stability and magnetic properties.
Draw the molecular orbital diagram for:(i) Be2(ii) B2 and ... (i) Be2 molecule: The electronic configuration of Be(Z = 4) is: 4 Be 1s 2 2s 1 Be 2 molecule is formed by the overlap of atomic orbitals of both beryllium atoms. Number of valence electrons in Be atom = 2 Thus in the formation of Be 2 molecule, two outer electrons of each Be atom i.e. 4 in all, have to be accommodated in various molecular orbitals in the increasing order of their energies. Molecular Orbital Diagram Be2 Draw the molecular orbital energy level diagram for each of the following species Be2+, Be2, and Be Indicate theirnumbers of unpaired electron and mention their magnetic diagramweb.netate their bond orders, and state which species is moststable% (1). Bond order of Be2 is A 1 B 2 C 3 D 0 class 11 chemistry CBSE Bond order is equal to half of the difference between the number of electrons in bonding (. N b. ) and antibonding molecular orbitals (. N a. ). Complete Solution : B e 2. molecule will be formed by the overlapping of atomic orbitals of two beryllium atoms. A Be atom has four electrons. Solved 1. Draw the molecular orbital energy level diagram ... 1. Draw the molecular orbital energy level diagram for each of the following species Be2+, Be2, and Be2-. Indicate theirnumbers of unpaired electron and mention their magnetic properties.Calculate their bond orders, and state which species is moststable. Expert Answer 91% (11 ratings) Atomic number of Be is 4 so it has 4 electrons .
34 Be2 Mo Diagram Wiring Diagram Database Dubai Burj ... How to make the molecular orbital diagram for be2: does the molecule exist? this video discusses how to draw the molecular orbital (mo) diagram for the be2 molecule. the bond order of be2 is calculated ncert problem 4.35 page no. 135 chemical bonding and molecular structure use molecular orbital theory to explain why the for the molecule be2: a) draw the molecular orbital diagram.
Why does the molecular orbital diagram for Be2+ consist of ... For completeMO diagrams, you use the total number of electrons. The 1s electrons of O2, N2, etc. are used to fill up the sigma(1s) and sigma(1s)* molecular orbitals. Similarly, with Be2+as well, there are 2(4) - 1 = 7 total electrons if you're filling out a complete MO diagram. 12 Share ReportSave level 2 Op· 3y
What is the bond order of Be2 −?... | Clutch Prep FREE Expert Solution. We're being asked to determine the bond order of Be2-. For this, we need to do the following steps: Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Step 3: Calculate the bond order of the molecule/ion. Recall that the formula for bond order is:
(Solved) - molecular orbital diagram. li2 is stable but ... molecular orbital diagram 1 answer below » li2 is stable but be2 is not stable . explain 1 Approved Answer Dimple D answered on December 17, 2020 4 Ratings, ( 15 Votes)
Molecular Orbital (MO) Diagram of Be2 - YouTube Molecular Orbital Diagram for Beryllium Dimer (Be2)Fill from the bottom up, with 4 electrons total.Bonding Order is 0, meaning it does not bond, and it is di...
What Is The Bond Order Of B2 1 So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = . 5 * (# of bonding electrons - # of antibonding electrons). However, when you draw the Lewis structure of B2, you get a triple bond. Nov 11, 2016
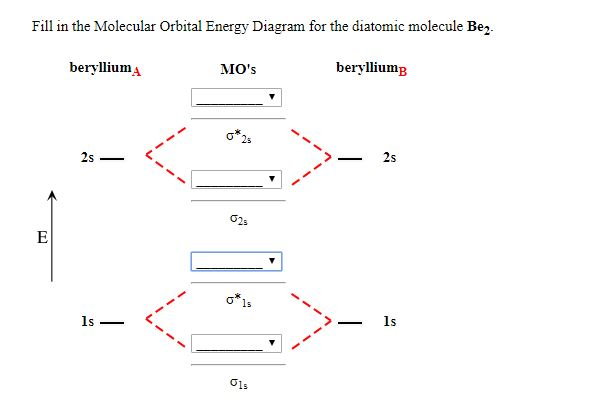
Solved Construct the molecular orbital diagram for Be2 ... Expert Answer 100% (24 ratings) Transcribed image text: Construct the molecular orbital diagram for Be2. Note that the 1s orbitals are not shown. Be Ho Be Answer Bank IL | Identify the bond order. O 0 O os O 1s Previous question Next question
Molecular Orbital Theory - Build Be2+ - YouTube For the ion Be2+:a) Draw the molecular orbital diagram.b) Calculate the bond order.c) Would this ion exist?d) Write the electron configuration of the ion————...
39 mo diagram of b2 - Wiring Diagram Images C22- Molecular Orbital Diagram B2 MO diagram with no sp mixing: B2 = 6 e⁻. Label the sigma bonding molecular orbitals on the diagram above using the designation. d. Give the electron configurations for the species C2 and C When we draw the C2 MO, we have everything up till the PiPy Orbitlal filled, and the next orbital tht would be filled ...
34 Be2 Mo Diagram Wiring Diagram Database - Dubai Burj ... this video discusses how to draw the molecular orbital (mo) diagram for the be2 molecule. the bond order of be2 is calculated ncert problem 4.35 page no. 135 chemical bonding and molecular structure use molecular orbital theory to explain why the for the molecule be2: a) draw the molecular orbital diagram. b) calculate the bond order.
Molecular Orbital Theory - Purdue University below. These molecular orbitals are called pi() orbitals because they look like porbitals when viewed along the bond. Whereasand * orbitals concentrate the electrons along the axis on which the nuclei of the atoms lie, and * orbitals concentrate the electrons either above or below this axis.
Be2 Molecular Orbital Diagram Nov 11, · As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. As discussed in class it is not a bond.
40 molecular orbital diagram for he2+ - Diagram For You So the bond order of B2 is equal to 1, which you can get by drawing the molecular orbital diagram and performing the equation Bond Order = . 5 * (# of bonding electrons - # of antibonding electrons). However, when you draw the Lewis structure of B2, you get a triple bond . On the basis of molecular orbital theory, explain why `He ...
Use molecular orbital theory to explain why Be2 molecule ... Use molecular orbital theory to explain why Be2 molecule does not exist. CLASSES AND TRENDING CHAPTER class 5 The Fish Tale Across the Wall Tenths and HundredthsParts and Whole Can you see the Pattern? class 6 Maps Practical Geometry Separation of SubstancesPlaying With Numbers India: Climate, Vegetation and Wildlife class 7


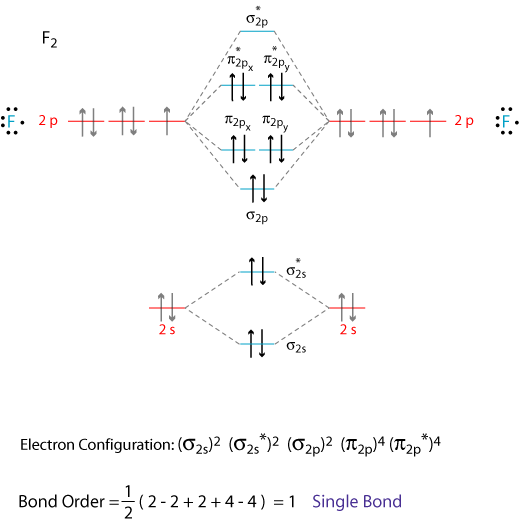




.bmp)

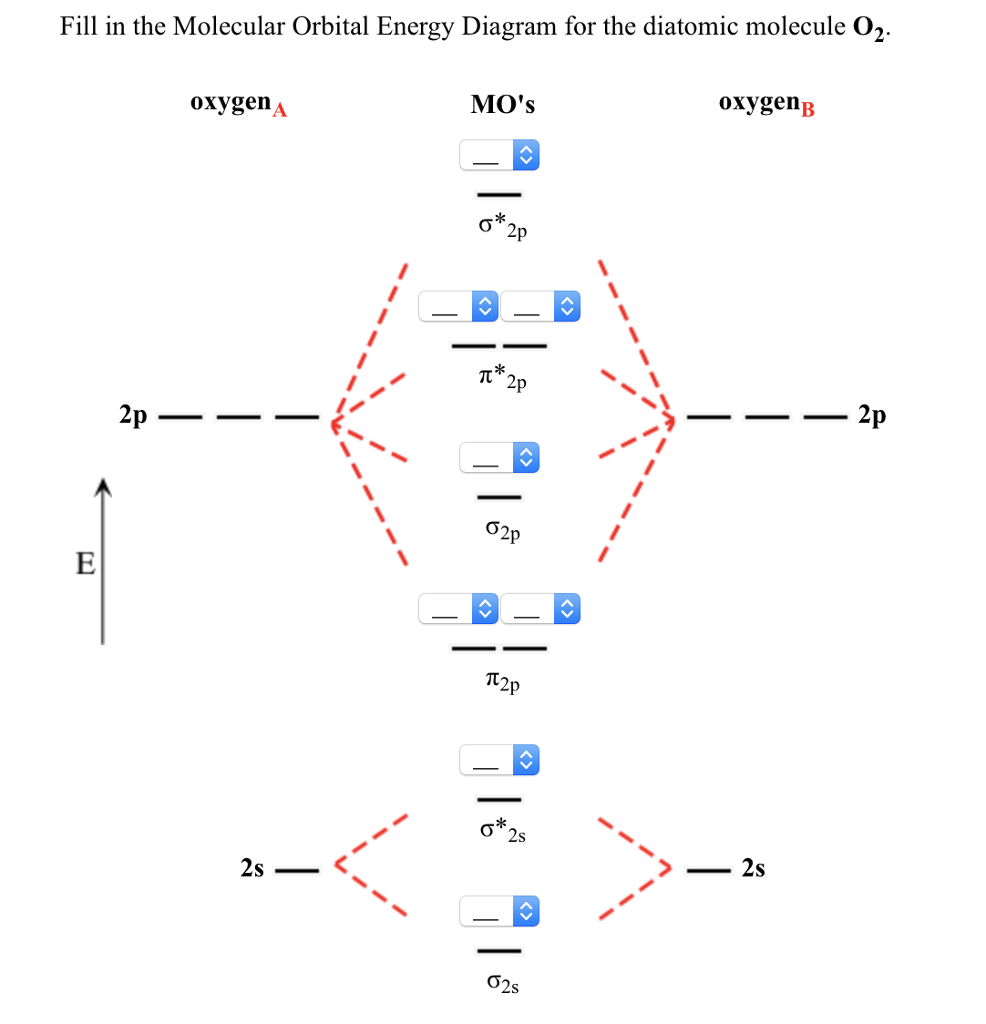




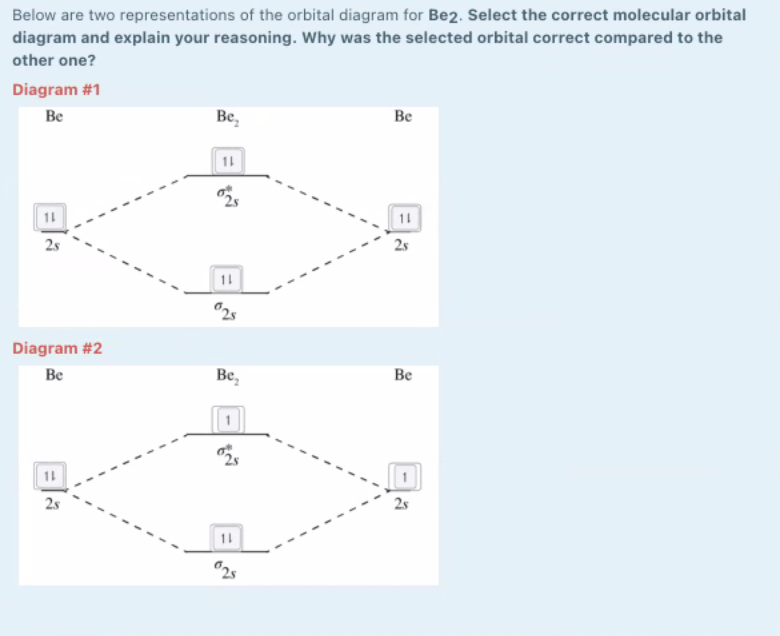









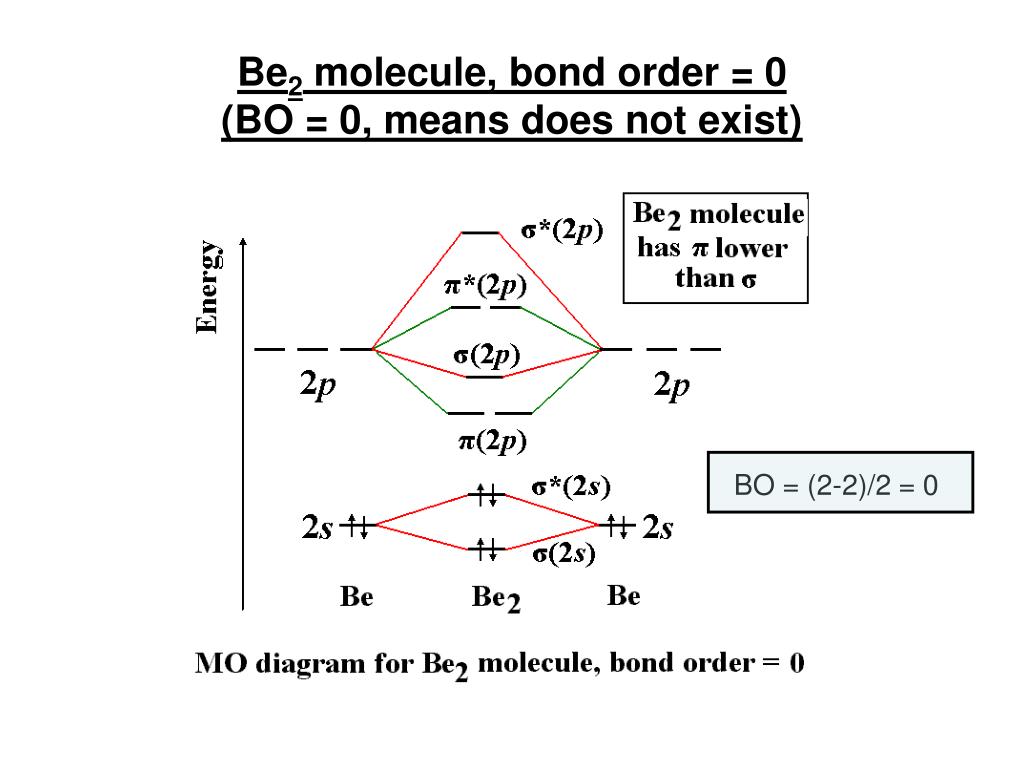



0 Response to "40 molecular orbital diagram for be2"
Post a Comment