42 the ammonia molecule in the diagram has the observed bond orientation because
An electrically neutral molecule has the formula C3H4O2N. If the carbon atoms form the usual number of bonds, how many covalent bonds will each hydrogen atom have with other atoms in the molecule? The type of bonding and the numbers of covalent bonds an atom can form ... The ammonia molecule in the diagram has the observed bond orientation because.
The Ammonia Molecule In The Diagram Has The Observed Bond Orientation Because ... 1jzgte Mk3 Supra Tachometer Wiring Diagram; Trrs Plug Wiring; Kenwood Kdc 1011s Wiring Diagram; Sensi St55 Wiring Diagram; 1999 Oldsmobile Intrigue Radio Wiring Diagram; Quadrajet Vacuum Ports Diagram; Baldor Cl3608tm Wiring Diagram; Tach Wiring Diagram For A 81 ...
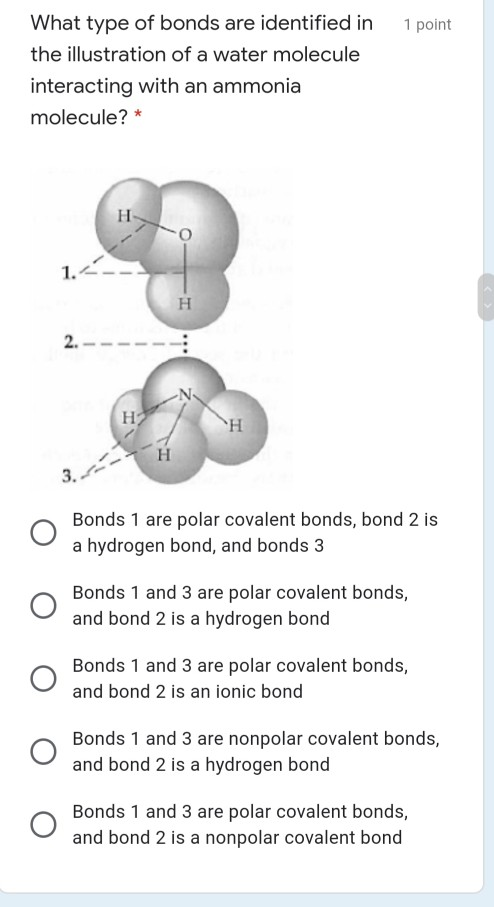
The ammonia molecule in the diagram has the observed bond orientation because
Molecular geometry. The theory of valency which we have been developing is known as valence bond theory.One further feature of this theory is that it may be used to predict (or in some cases, rationalize) the observed geometries of molecules By the geometry of a molecule we mean the relative arrangement of the nuclei in three-dimensional space. By making two covalent bonds, an O atom with 8. Skip to main content ... the pictured (the only attachrd photo) molecule can change its shape because: a. some atoms make longer bonds than others ... d. electrons can move from one bond to another. e. none of the above. 5. The ammonia molecule in the below diagram has the observed orientation ... Nitrogen has a total of 7 protons (its atomic number is 7) in its nucleus. Explanation: The shape and the bond orientation of molecules and ions are both explained by the valences shell electron pair repulsion theory (VSEPR). Ammonia, , is a molecule which contains three N-H bonds, as well as one lone pair on nitrogen. According to the VSEPR ...
The ammonia molecule in the diagram has the observed bond orientation because. The ammonia molecule in the diagram has the observed bond orientation because... a) electrons repel one another b) N has four pairs of electrons in the valence shell c) N has 7 protons in its nucleus d) all of the above e) none of the above The ammonia molecule (NH3) in the diagram has the observed bond orientation because ... a. N has four pairs of electrons in the valence shell. b. N has 7 protons in its nucleus. c. electrons repel one another. d. All of the above. e. None of the above. Ammonia, , is a molecule which contains three N-H bonds, as well as one lone pair on nitrogen. According to the VSEPR theory, molecules try to acquire a shape which would minimize the repulsion exhibited by the electron clouds present, that is, between the bonding (shared in a bond) and non-bonding (lone pair) electrons. Part F The ammonia molecule in the diagram has the observed bond orientation because ... ANSWER: The ammonia molecule in the diagram has the observed bond orientation because ... N has 7 protons in its nucleus. N has four pairs of electrons in the valence shell. electrons repel one another. All of the above. None of the above.
(a) Each CO bond has a bond dipole moment, but they point in opposite directions so that the net CO 2 molecule is nonpolar. (b) In contrast, water is polar because the OH bond moments do not cancel out. The OCS molecule has a structure similar to CO 2, but a sulfur atom has replaced one of the oxygen atoms. To determine if this molecule is ... This preview shows page 1 - 3 out of 4 pages. The ammonia molecule in the diagram has the observed bond orientation because…. All of the above (N has 7 protons in its nucleus, electrons repel one another, N has four pairs of electrons in the valence shell) The discovery of which of the following led to a drastic change in deep sea mining? Thus far, we have used two-dimensional Lewis structures to represent molecules. However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space ().A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. To obtain a more accurate expression of the rate for the special case of ammonia molecule, we observed geometry optimization which leads to N-H bond length of 1.017 Å, while H-N-H bond angle is ...
The ammonia molecule in the diagram has the observed bond orientation because ... All of the above. Without making or breaking bonds, the pictured molecule can change its shape because ... rotation can occur around single bonds. The ammonia molecule in the diagram has the observed bond orientation because ... All of the above a. N has four pairs of electrons in the valence shell The ammonia molecule in the diagram has the observed bond orientation because ... N has four pairs of electrons in the valence shell. N has 7 protons in its ... The ammonia molecule in the diagram has the observed bond orientation because … N has 7 protons in its nucleus. N has four pairs of electrons in the valence shell. electrons repel one another. All of the above. None of the above. All of the above. Without making or breaking bonds, the pictured molecule can change its shape because …
Alpine 445u Wiring Diagram; Isuzu I290 Engine Wiring Diagram; Ge Gss22wg Wiring Diagram; Cadette Sash Diagram; Intermatic 240v Timer Wiring Diagram; The Ammonia Molecule In The Diagram Has The Observed Bond Orientation Because ... Ouku Radio Wiring Diagram; Dual Xhd6425 Wiring Harness; Recent Comments. Brad H. on Lutron three way switch wiring ...
The ammonia molecule in the diagram has the observed bond orientation because... a) electrons repel one another b) N has four pairs of electrons in the valence shell c) N has 7 protons in its nucleus d) all of the above e) none of the above
Covalent bonds hold atoms together because they... fill shells without giving atoms much charge ... The ammonia molecule in the diagram has the observed bond orientation b/c? all of the above ... an electrically neutral molecule has the formula C3H4O2N. If the carbon atoms from the usual number of bonds, how many covalent bonds will each ...
The Ammonia Molecule In The Diagram Has The Observed Bond Orientation Because ... Sony Gt90bt Wiring Diagram; Cat No Ig13cp Wiring Diagram; 1994 Zx7 Wiring Diagram; 782-1768 Wiring Diagram; Kohler Cv730s Engine Diagram; Hastings Cvt Wiring Diagram; Ahe60d3xh21 Wiring Diagram; Homa Pumps Wiring Diagram; Stinger Sgp38 80-amp Battery Isolator And ...
The ammonia molecule in the diagram has the observed bond orientation because ... A. N has four pairs of electrons in the valence shell. B. electrons repel one another. C. N has 7 protons in its nucleus. D. All of the above. E. None of the above.
Covalent Bonds hold atoms together because they.. ... an atoms atomic number is 7. Its valence is most likely. 3. By making two covalent bonds, an O atom (w/ 8 protons) fills its valence shell. ... In a double covalent bond, a carbon atom shares.. electrons in two orbitals. The ammonia molecule in the diagram has the observed bond orientation ...
The ammonia molecule in the diagram has the observed bond orientation because a. (c) The electrostatic potential diagram of the water molecule. The polarity of the NOH bonds occurs because nitrogen has a greater electronegativity than hydrogen. (b) The dipole moment of the ammonia molecule oriented in an electric field.
The ammonia molecule in the diagram has the observed bond orientation because ... There is a ball-and-stick model of ammonia, NH3. Three hydrogen atoms are attached to nitrogen. N has 7 protons in its nucleus. N has four pairs of electrons in the valence shell. All of the above. None of the above.
The process of chemisorption of small molecules on transition metal surfaces has been investigated from the point of view of two prototype adsorption systems, carbon monoxide on iridium {100} and {111 } and ammonia on nickel {111}. Microscopic details such as the molecular orientation on the surface, the bonding configuration and the nature of interactions between adsorbed species have been ...
Nitrogen has a total of 7 protons (its atomic number is 7) in its nucleus. Explanation: The shape and the bond orientation of molecules and ions are both explained by the valences shell electron pair repulsion theory (VSEPR). Ammonia, , is a molecule which contains three N-H bonds, as well as one lone pair on nitrogen. According to the VSEPR ...
By making two covalent bonds, an O atom with 8. Skip to main content ... the pictured (the only attachrd photo) molecule can change its shape because: a. some atoms make longer bonds than others ... d. electrons can move from one bond to another. e. none of the above. 5. The ammonia molecule in the below diagram has the observed orientation ...
Molecular geometry. The theory of valency which we have been developing is known as valence bond theory.One further feature of this theory is that it may be used to predict (or in some cases, rationalize) the observed geometries of molecules By the geometry of a molecule we mean the relative arrangement of the nuclei in three-dimensional space.

0 Response to "42 the ammonia molecule in the diagram has the observed bond orientation because"
Post a Comment