41 tin electron dot diagram
To write the configuration for the Tin (Sn) and the Tin ions, first we need to write the electron configuration for just Tin (Sn). We first need to find the ... Atomic Structure Links · Valence Electrons and Lewis Electron Dots of Atoms and Ions
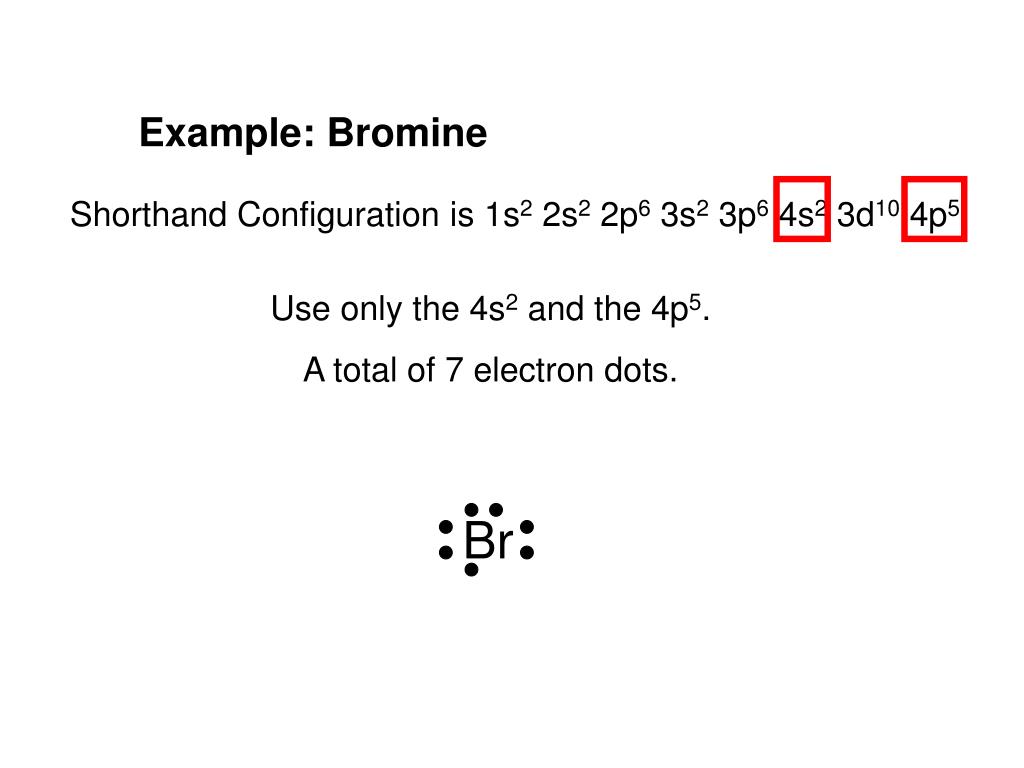
June 4, 2018 - Electron dot diagrams, sometimes called Lewis dot diagrams, were first used by Gilbert N. Lewis in 1916. These diagrams are used as a shorthand notation to show the number of valence electrons in an atom.
Tin electron dot diagram
Learn about the chemistry topic Notation: Orbital and Lewis Dot in this free and fun science study guide! We answer the basic questions and break it down in an easy-to-understand format. This is the correct dot diagram for sodium, group 1. Q. This is the correct dot diagram for nitrogen, group 15. Q. This is a correct dot diagram for neon, group 18. Q. This could be the dot diagram of. Mg, group 2. Cl, group 17. 26.02.2018 · Green light-emitting diodes with a brightness of 460,000 cd m–2 and a low turn-on voltage of 2.5 V are enabled by the use of a chlorination treatment to provide conductive passivation of the ...
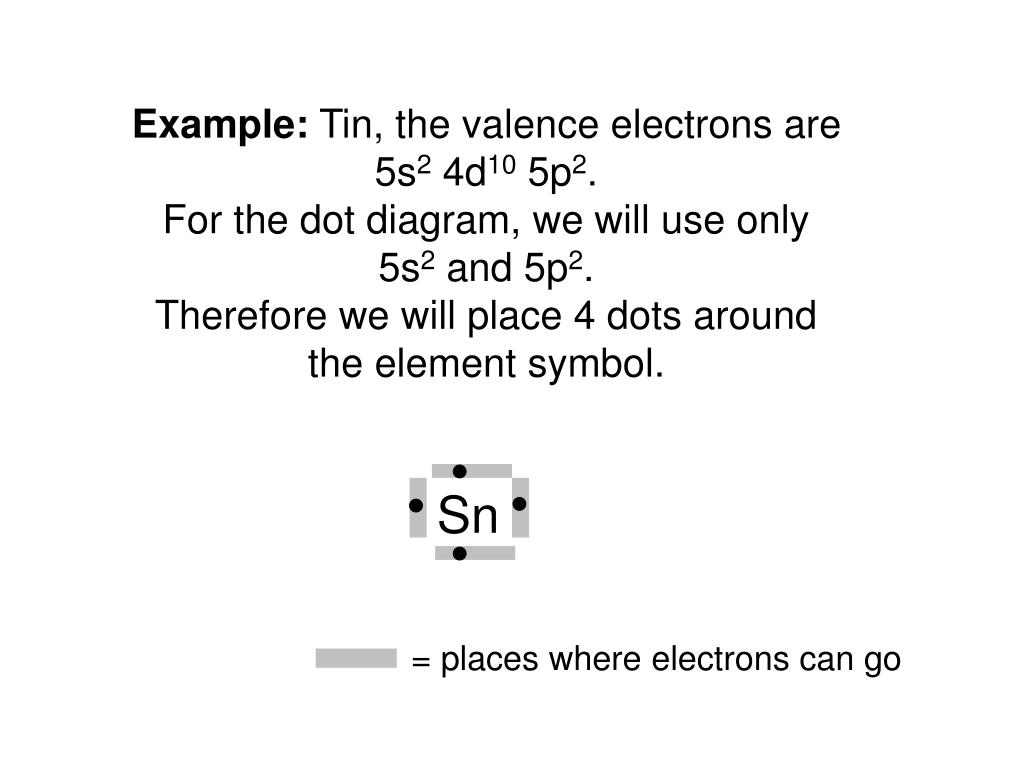
Tin electron dot diagram. First, we write the electron configuration and see how many valence electrons the element has, from there we can draw the electron-dot structure. electron-dot diagram of an atom of an element in Period 2 of the Periodic Table? ... toothpaste is tin(II) uoride. A town located downstream from a chemical plant was concerned about uoride ions from the plant leaking into its drinking water. According to the Environmental Protection Agency, the The main uses of stannic chloride are as a raw material for the manufacture of other tine compounds, especially organotins. and in the surface treatmentof glass and other nonconductive materials, whereby stannic oxide is deposited from the stannic chloride solutions onto the surface giving it strength, abrasive resistance, and conductivity. It is also widely used as a catalyst in Friedel-Craft ... Write electron dot diagrams for the following atoms: a. silicon b. rubidium c. barium d. tin e. iodine f. arsenic . Si Rb Ba Sn I As . 4 dots, 1 on 1 dot 2 dots, 1 4 dots, 1 7 dots, 2 dots on 5 dots, 2 dots on 1 side, each side each on 2 sides on each side 3 sides, 1 dot on 1 dot on each of the ...
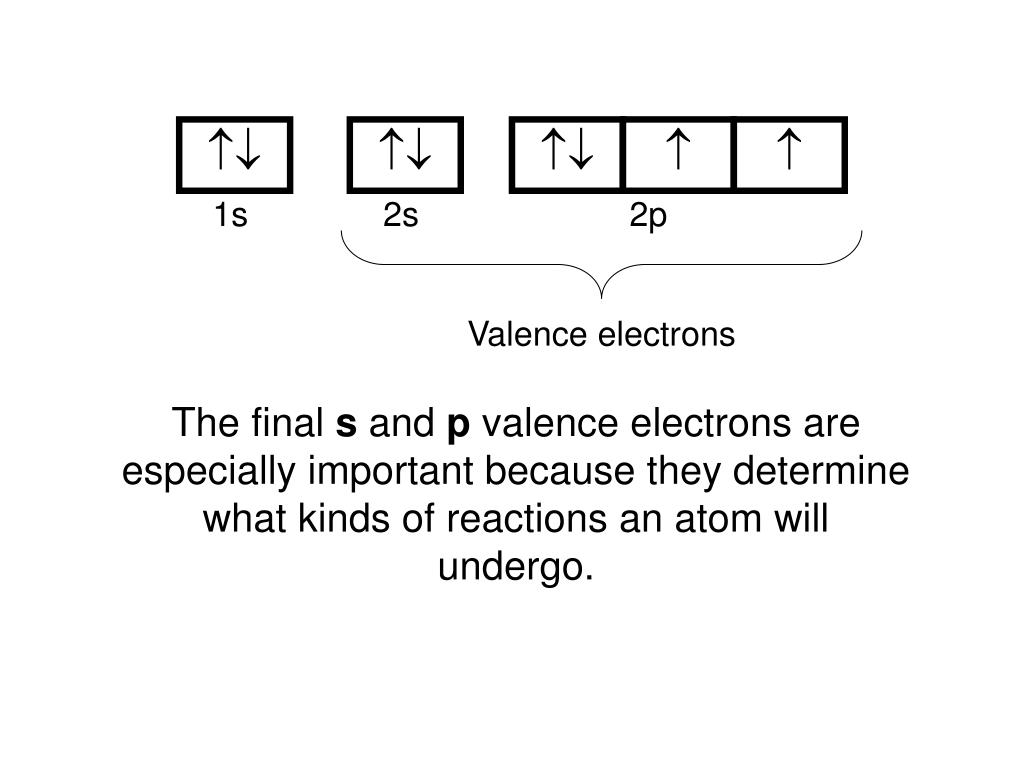
Includes Concise Form of Electron Configuration Notation and Tin (Sn). Githy.com. Multiple-site search is loading. Home Money Science & Tech U.S. World Environment Page-of-the-day Trivia Tin (Sn), Electron Configuration: [Kr] 4d 10 5s 2 5p 2 [Kr] 4d 10 5s 2 5p 2::: example of ... October 4, 2021 - Lewis used simple diagrams (now called Lewis diagrams) to keep track of how many electrons were present in the outermost, or valence, shell of a given atom. The kernel of the atom, i.e., the nucleus … Electron dot structures or Lewis dot formula can be drawn if the molecular formula of the compound is known. It defines the nature of bond and position of atoms of the molecule which are connected in the molecule. Electron dot structure Cyclopentane. In the cyclic form of cyclopentane, single bonds connect to five carbon atoms. A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
A solar cell, or photovoltaic cell, is an electrical device that converts the energy of light directly into electricity by the photovoltaic effect, which is a physical and chemical phenomenon. It is a form of photoelectric cell, defined as a device whose electrical characteristics, such as current, voltage, or resistance, vary when exposed to light. Outer Shell Electron Configuration: Electron Dot: c. Antimony: Outer Shell Orbital Notation: Outer Shell Electron Configuration: Electron Dot: 2. For each of the following ions, show the orbital notation (regular), electron configuration (regular), and electron dot picture: a. Aluminum (+3): Regular Orbital Notation: Regular Electron Configuration: For atoms with partially filled d or f subshells, these electrons are typically omitted from Lewis electron dot diagrams. For example, the electron dot diagram for iron (valence shell configuration 4s 2 3d 6) is as follows:. Elements in the same column of the periodic table have similar Lewis electron dot diagrams because they have the same valence shell electron configuration. Lewis Dot Diagrams of Selected Elements. Lewis Symbols: Electron Configuration into Shells: Index Chemical concepts Chemistry of the Elements Periodic Table . HyperPhysics***** Quantum Physics : R Nave: Go Back: Electron Distributions Into Shells for the First Three Periods. A chemical element is identified by the number of protons in its ...
f Configurational diagram for charge transfer between two nanocrystals, where an electron (black dot) moves from the nanocrystal on the left (configuration of reactants, Q R, with ground state ...
atomic symbol. The seventh electron is drawn as a sin-gle dot and is called an unpaired electron. Two fl uorine atoms can share their unpaired electrons and form a covalent bond. We can show this by means of a Lewis diagram as follows: Lets now take an example of an atom with more than one valence electron. The fl uorine atom has seven
Drawing Lewis Dot Symbols or Electron Dot Diagrams is an important skill in understanding molecular geometry and ionic crystals. It also helps with understan...
August 22, 2020 - Mentioned in the Hebrew scriptures, tin is of ancient origins. Tin is an element in Group 14 (The carbon family) and has mainly metallic properties. Tin has atomic number 50 and an atomic mass of 118.…
electron dot diagram do not represent all of the electrons in an atom, just the valence electrons. Have students look at Figure 2. Ask them to predict the electron dot diagrams for rubidium, strontium, indium, tin, anti-mony, tellurium, iodine, and xenon.
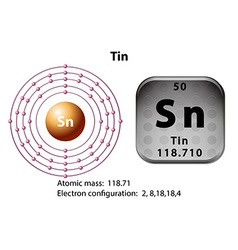
Tin atoms have 50 electrons and the shell structure is 2.8.18.18.4. The ground state electron configuration of ground state gaseous neutral tin is [ Kr ]. 4d10. 5s2. 5p2 and the term symbol is 3P0. Schematic electronic configuration of tin. The Kossel shell structure of tin. Atomic spectrum A representation of the atomic spectrum of tin.
October 4, 2021 - The bonding between atoms in a molecule can be topically modeled though Lewis electron dot diagrams. Creating Lewis diagrams is rather simple and requires only a few steps and some accounting of the …
Dec 26, 2021 · Electron dot structures of co2 determine the nature of bonds and atoms in molecule connected by it. Carbon dioxide is a colorless and odorless gas found naturally. CO2 is the chemical compound’s formula. Electron dot structures of co2 When first learning about Lewis structure and Molecular Geometry, an excellent place to start is with Carbon Dioxide, first-time students who ...
April 2, 2021 - When examining chemical bonding, it is necessary to keep track of the valence electrons of each atom. Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, has the ...
A Lewis Dot Structure is the diagrammatic representation of the bonding between the atoms of a molecule and the lone pair of electrons present in it. It is also known as electron dot structure/ Lewis dot diagram, Lewis dot formulas, Electron dot structure, or Lewis Electron dot structure (LEDs), respectively.
Tin (2+) difluoride | F2Sn - PubChem. National Center for Biotechnology Information. 8600 Rockville Pike, Bethesda, MD, 20894 USA. Contact. Policies. FOIA. National Library of Medicine. National Institutes of Health. Department of Health and Human Services.
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ...
November 25, 2020 - Sn Tin Element information, facts. Tin properties, uses and trends | Periodic Table of the Elements - complete information about the tin element - Facts, atomic mass, melting point, How to Locate on Periodic Table, History, Abundance, Physical Properties, Thermal Properties, Crystal Structure, ...
A Lewis electron dot diagramA representation of the valence electrons of an atom that uses dots around the symbol of the element. (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element.
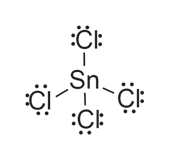
So the dot structure of tin (IV) would have Sn (symbol) in the middle (since it is more electronegative). Now, indicate the valence electrons by putting 4 dots around it. Iodine wants to gain 1 electron, so it has 7 valence electrons. So simply write I and put 7 dots around it.
The following lesson looks at drawing electron dot diagrams. Download the following lesson for free from iTunes by typing in the search window "PapaPodcasts"...
21. sep. 2018 ... Tin Electron Configuration: Tin is a chemical element that has the symbol Sn (It is taken from the Latin word tannum).
Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.. The electron configuration for the first 10 elements. H #1s^1# He #1s^2# Li #1s^2 2s^1# Be #1s^2 2s^2# B #1s^2 2s^2 2p^1# C #1s^2 2s^2 2p^2# N #1s^2 2s^2 2p^3# O #1s^2 2s^2 2p^4# F #1s^2 2s^2 …
3. When writing the electron configuration of an atom, in what general order are the sublevels written? 4. How is the number of electrons in an energy sublevel indicated in an electron configuration? 5. What is the electron configuration for tin (Sn)? 6. The valence orbitals in an atom are the _____. 7. What is an electron dot structure? 8.
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element’s symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
Lewis symbols (also known as Lewis dot diagrams or electron dot diagrams) are . Neon (Ne), argon (Ar), krypton (Kr), etc., each contain eight electrons in their. Column 1A 1 valence electron The first symbol in the column is H . electrons and draw the Lewis dot structure. 1. Barium 6. Carbon. 2. Tin 7. Krypton. 3.
Vi ville gerne vise dig en beskrivelse her, men det websted, du kigger på, tillader det ikke.
A step-by-step explanation of how to draw the SnF2 Lewis Dot Structure.For the SnF2 structure use the periodic table to find the total number of valence elec...
Tin electron configuration ; Electronic configuration of the Tin atom in ascending order of orbital energies: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p ...
Electron dot formula shows the number of valence electrons for that element with the help of dots. The valence electrons are those electrons that occupy the highest energy level. We can obtain it by using the periodic table. For example, the elements in group IA of the chemical periodic table have 1 valence electron.
Write electron configuration and draw energy level diagram, Bohr Rutherford diagram and Lewis's dot diagram for each of the following atom /ion. a) Bismuth (Bi) Atomic #50. b) Calcium ion (Ca++) Atomic # of Calcium atom is 20. c) Tin (Sn) Atomic #50. Expert Answer.
Electrons Per Shell, 2 8 18 18 4. Electron Configuration, [Kr] 4d10 5s2 5p2. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p2. Orbital Diagram.
Lewis. These visual representations were given the name Lewis electron dot diagrams. Lewis suggested that since the valence electrons are responsible for chemical reactions and the core electrons are not involved, we should use a diagram that shows just the valence electrons for an atom.
Lewis Dot Diagrams Name Verónica Borrego Quezada Chem Worksheet 5-7 The outermost electrons in an atom are called the valence electrons. These electrons are especially important because they interact with other atoms in chemical bonds. ... When the electron configuration is known, the number of valence electrons can be determined by adding up ...
A step-by-step explanation of how to draw the Arsenic (As) Lewis Dot Structure.For the ArsenicLewis structure use the periodic table to find the total number...
The quick answer here is that because tin, #"Sn"#, is a main-group element, the number of valance electrons will be given by its group number.. Tin is located in group #1color(red)(4)# of the periodic table, which means that it has #color(red)(4)# electrons in its outermost shell, i.e. #color(red)(4)# valence electrons.. Now, you can prove that this is the case by constructing tin's electron ...
Which is the ending electron configuration of tin? 5p2. How many dots would appear on the Lewis electron dot diagram for an atom whose electron notation ends in 6s25d106p4? 6. Element X has an electron configuration that ends with 4p4. How many electrons would it like to gain to achieve stability? 2.
Negative Electric Charge Electron Dot Diagram Polar Covalent Bond Covalent Bond Physical Science. TERMS IN THIS SET (14) electron dot diagram. a model of an atom in which each dot represents a valence electron. ion. an atom that has a net positive or negative electric charge. anion. an ion with a negative charge.
Problem 93 Medium Difficulty. For an atom of tin in the ground state, write the electron configuration using noble-gas notation, and draw its electron-dot structure.
Comprehensive data on the chemical element Tin is provided on this page; including scores of properties, element names in many languages, most known nuclides of Tin. Common chemical compounds are also provided for many elements. In addition technical terms are linked to their definitions and ...
The electron dot diagram is a way of working out the bonding of a molecule. The electron cloud is a description of the way that electrons surround the nucleus. The definition of electron dot...
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
The telephone emerged from the making and successive improvements of the electrical telegraph.In 1804, Spanish polymath and scientist Francisco Salva Campillo constructed an electrochemical telegraph. The first working telegraph was built by the English inventor Francis Ronalds in 1816 and used static electricity. An electromagnetic telegraph was created by Baron …
26.02.2018 · Green light-emitting diodes with a brightness of 460,000 cd m–2 and a low turn-on voltage of 2.5 V are enabled by the use of a chlorination treatment to provide conductive passivation of the ...
This is the correct dot diagram for sodium, group 1. Q. This is the correct dot diagram for nitrogen, group 15. Q. This is a correct dot diagram for neon, group 18. Q. This could be the dot diagram of. Mg, group 2. Cl, group 17.
Learn about the chemistry topic Notation: Orbital and Lewis Dot in this free and fun science study guide! We answer the basic questions and break it down in an easy-to-understand format.















0 Response to "41 tin electron dot diagram"
Post a Comment