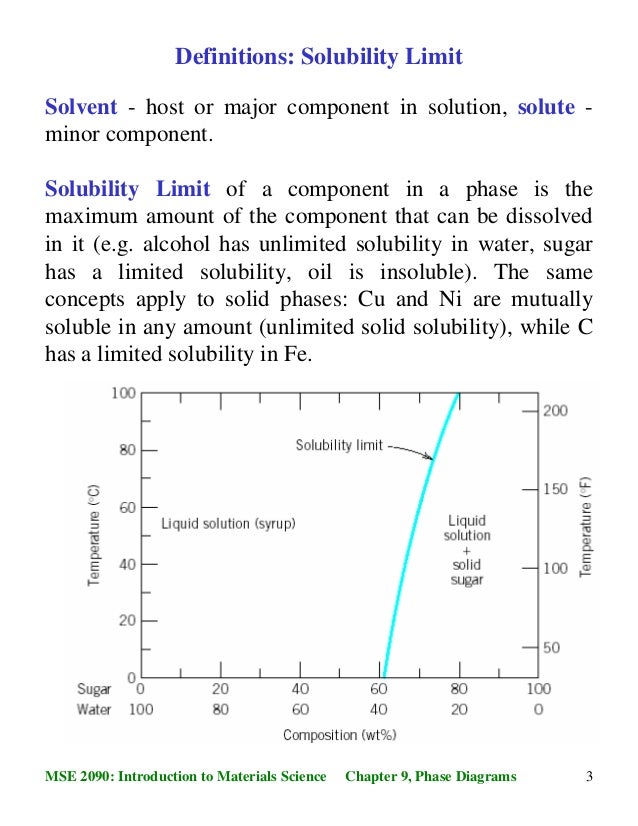
41 incongruent melting phase diagram
phase condensed system. ´The system consists of four curves and three points. 1.The curve AB (The melting point curve of ice) A is the melting point of ice, curve RS shows the lowering offf melting point of ice on the addition of anhydrous sodium- sulphate. ´ 5D.4(a) Indicate on the phase diagram in Fig. 5.4 (Fig. 5.3, 10th; Fig. 5.66, 9th) the feature that denotes incongruent melting. What is the composition of the eutectic mixture and at what temperature does it melt? Fig. 5.4: 5D.4(b) Indicate on the phase diagram in Fig. 5.5 (Fig. 5.4, 10th; Fig. 5.67, 9th) the feature that denotes incongruent ...
Download scientific diagram | 1.: Example of a binary phase diagram with an incongruent melting phase γ. Inset: The distribution coefficient is set constant by assuming straight liquidus and ...

Incongruent melting phase diagram
The phase diagram has four maxima corresponding to the formation of four hydrates. Point C (37°C). E(32.5°C) and I(78.5°C) represent the congruent melting point of dodeca, hepta, penta and tetra hydrates respectively. The congruent melting point of a salt hydrate is also known as the dyestectic point. (G) Copper sulphate water vapour system The incongruent melting behavior results in an obvious decrease in crystallinity, leading to the lowest emission intensity. As mentioned above, the preferred orientation of Cs 3 Cu 2 Br 5 samples changes with the calcination temperature, and this result may also account for the difference in emission intensity. Therefore, this work highlights ... It showed all their developmental products
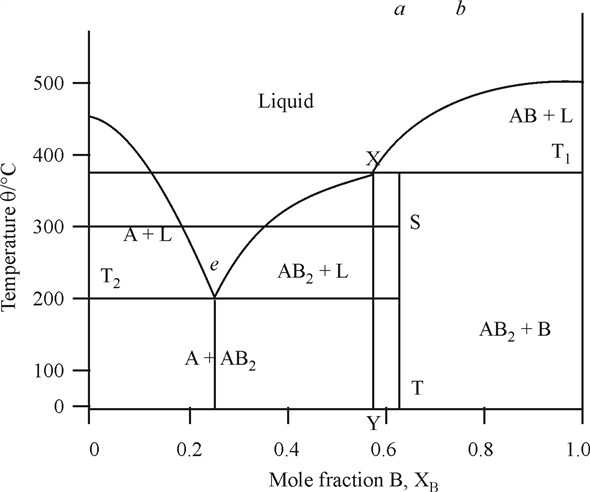
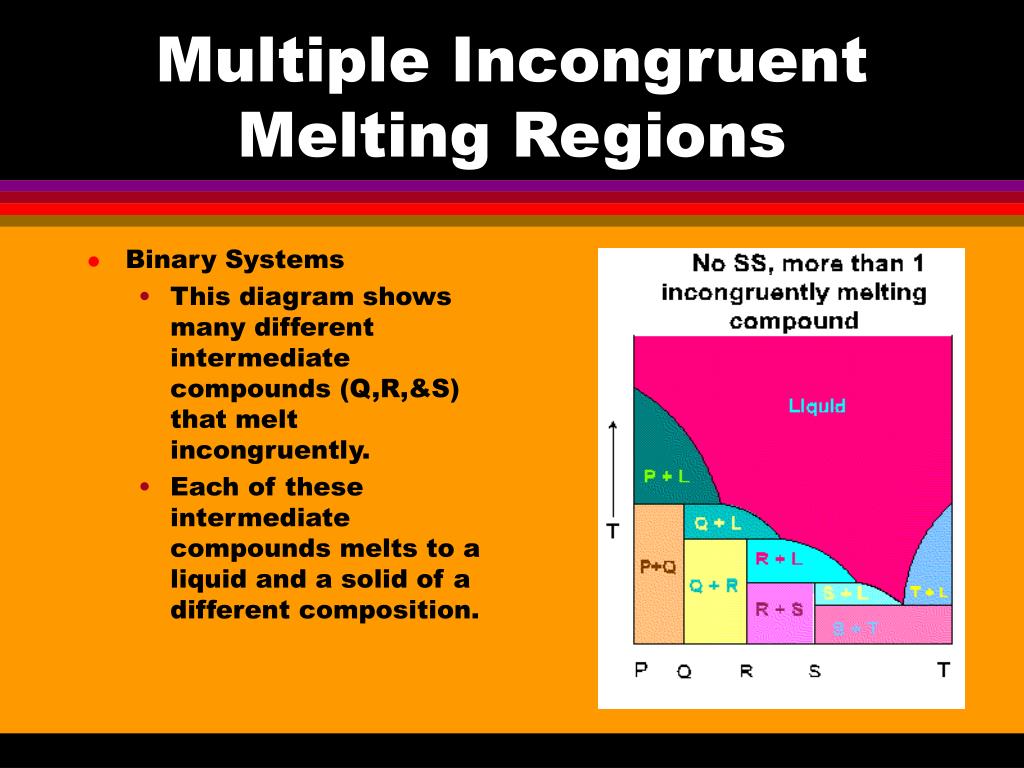
Incongruent melting phase diagram. • Incongruent melting is the temperature at which one solid phase transforms to another solid phase and a liquid phase both of different chemical compositions than the original composition. • This can be seen in this diagram as XY2 melts to Y and liquid. f Multiple Incongruent Melting Regions Binary Systems • This diagram shows many different Simple Ternary Phase Diagram Igneous and Metamorphic Petrology Q-Ab-Or system at high PH2O After Philpotts, 1990 1. Label the fields and any peritectics or eutectics on the diagram. 2. Draw the arrows on the cotectics or reaction lines. 3. What is the first phase to crystallize from composition X? (Label the diagram.) 4. what is the shape of the phase diagram of a liquid mixture containing two compounds forming AB and A2B structural solids/lattice rather than forming A and B solids/lattice when melted? Phase diagrams are from ASM Alloy Phase Diagram Database 2. 3 Chang 2016 Metal chalcogenides family 2D Josephson Junction NbSe 2 NbSe 2 Liu 2019 SC/TI proximity topological qubits ... •Incongruent or high melting temperature => bulk crystal growth demonstrated: SnSe 2, MoSe 2, WSe 2, Fe 3
12.104 Using Phase Diagrams Some useful terminology: Liquidus – region above which liquid is the only stable phase for the entire system Solidus – region below which solids are the only stable phases in the system The lever rule – For a given bulk composition you use the lever rule to calculate the amount of the phase that will be present in a two-phase assemblage. A ChemE freshman here, needing help. Would like to plot on screen first so my worksheet would be neat. Thanks!! Phase diagrams commonly contain intermediate compounds. There are both incongruent and congruent melting compounds. An incongruent melting point will end in ... I'm building a custom junction box for a friend. He wants stereo outputs to his amps, but wants a phase inverter switch for the second amp. Not quite sure how to do it! Can anyone help?
Hi, ​ I started diagnosing a problem where my acceleration suddenly stopped working (mid ride) and instead slows down when pressing the accelerator (also makes strange "rattling" noise): [https://imgur.com/a/4H5FZa2](https://imgur.com/a/4H5FZa2) ​ After taking things apart I found that my phase cable connectors are melted: [https://imgur.com/a/G1uiPih](https://imgur.com/a/G1uiPih) ​ Any idea if this is the cause and I can fix it by soldering the wires, or... Congruent melting - melting wherein a phase melts to a liquid with the same composition as the solid.Incongruent melting - melting wherein a phase melts to a liquid with a composition different from the solid and produces a solid of different composition to the original solid.. Why is the triple point of water so unique? The triple point is suitable because it is unique, i.e., it occurs at one ... Mantle Melting and Phase Diagrams William Wilcock ... SiO2 Phase diagram Forsterite and enstatite undergo incongruent melting En Fo + Liquid Mantle "composition" Effects of pressure on melting of Forsterite - Enstatite mixtures Surface 15 km >15 km Key Point - At depth of mantle melting, melt composition is somewhat pressure dependent ... 2212 phase is not a line compound but has a homogeneity range of 2.1 ^ Bi < 2.3 and 2.05/.95 > Sr/Ca ^ 1.6/1.4. A possible reason why many groups observe different phases during the incongruent melting and solidification is that their starting Bi2Sr2CaCu208 compositions are different. The specific goals of this project are divided into two steps.
I’d like to wire one of my guitars with 2 humbuckers with a volume and tone for each, a 3 way pickup selector switch, a 2 way switch to go out of phase, and a 2 way switch to split the coils. Like the wiring on the frank zappa Roxy SG.
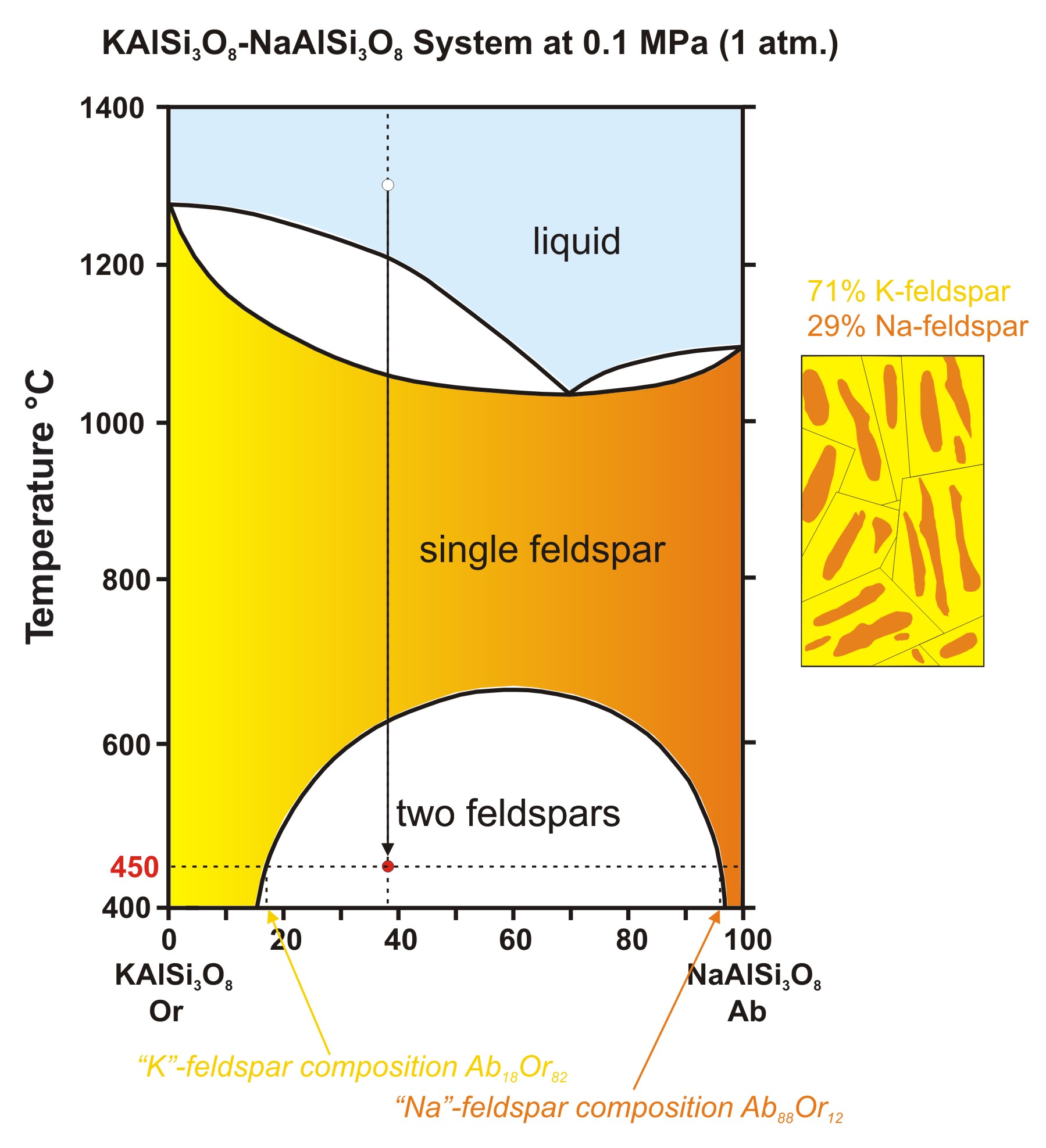
Two component Na/K phase diagram showing incongruent melting compound Na2K and amalgams (solid phase solutions) of Na in K or of K in Na. This final phase diagram shows a two component system made up of Na and K metals in different compositions. In this you can identify the incongruent melting compound and there are two new areas on either side ...
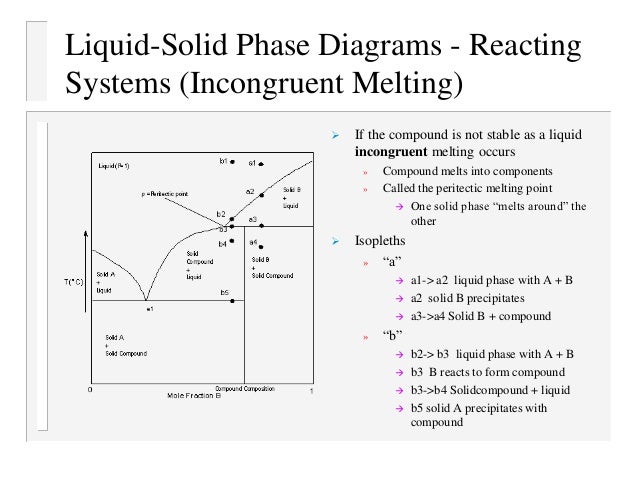
Phase equilibria and phase diagrams ... Incongruent melting: A crystalline phase melts into a solid phase (peritectic phase) and a liquid.37 pages
(a) Label the regions of the phase diagram. (b) Indicate on the phase diagram the feature that denotes incongruent melting. (c) Indicate on the phase diagram the composition of the eutectic mixture and temperature it melts? b T, Temperature, T T2 т. 0.2 0.8 1 0.4 0.6 Mole fraction of B, X,
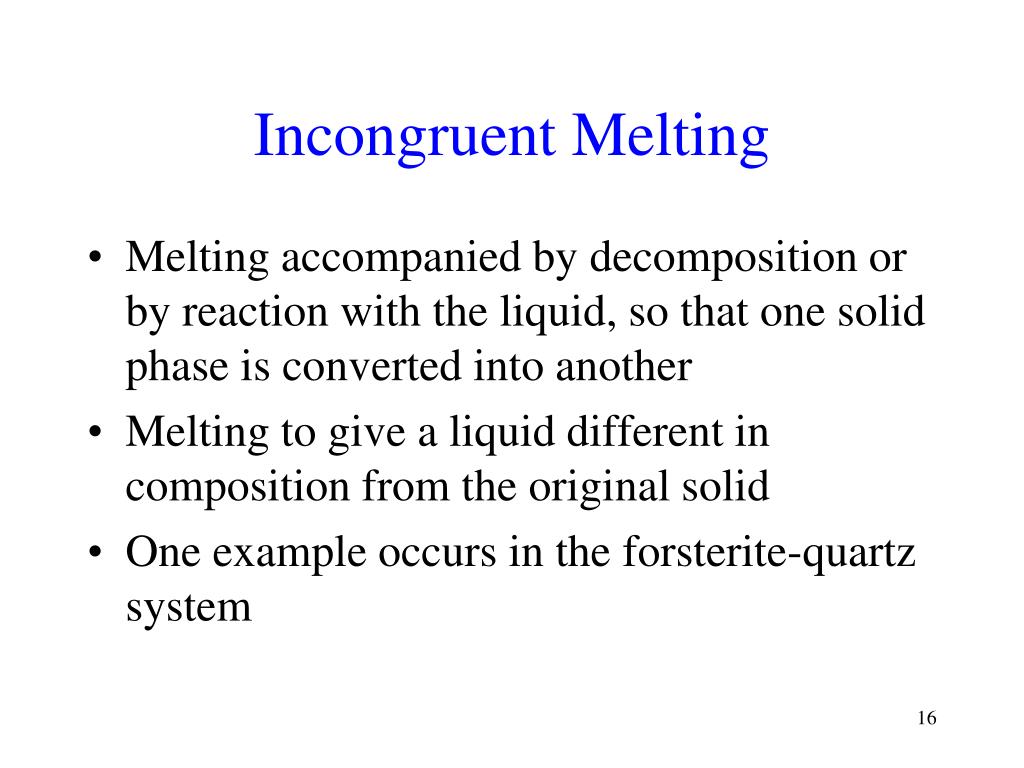
When a solid substance does not melt uniformly. Incongruent melting occurs when a solid substance does not melt uniformly, so that the chemical composition of the resulting liquid is not the same as that of the original solid. During incongruent melting a new solid of different composition forms. For example, melting of orthoclase (KAlSi 3 O 8) produces leucite (KAlSi 2 O 6) in addition to a melt.
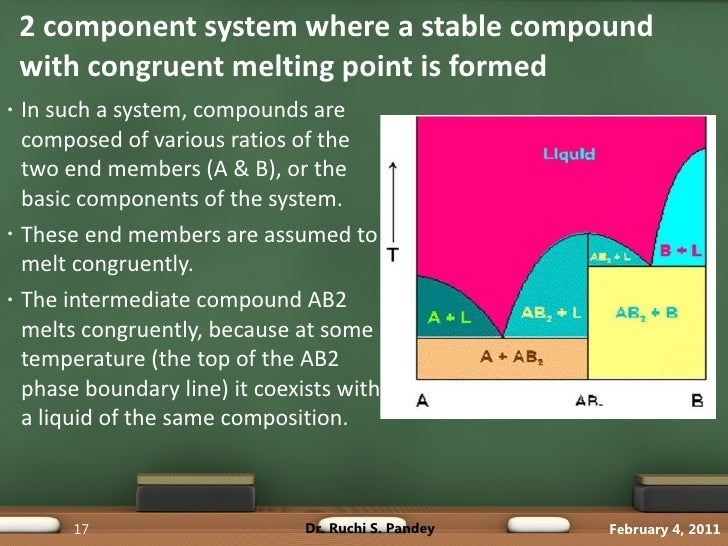
Figure 5. General phase diagram of eutectic systems. 2. Systems forming solid compounds AxBy with congruent and incongruent melting points: In these types of systems, the components do react with each other and the formation of a compound takes place. The common example of such systems are the Mn-Zn system (forming MgZn2) and Na2SO4-H2O system ...
Academia.edu is a platform for academics to share research papers.
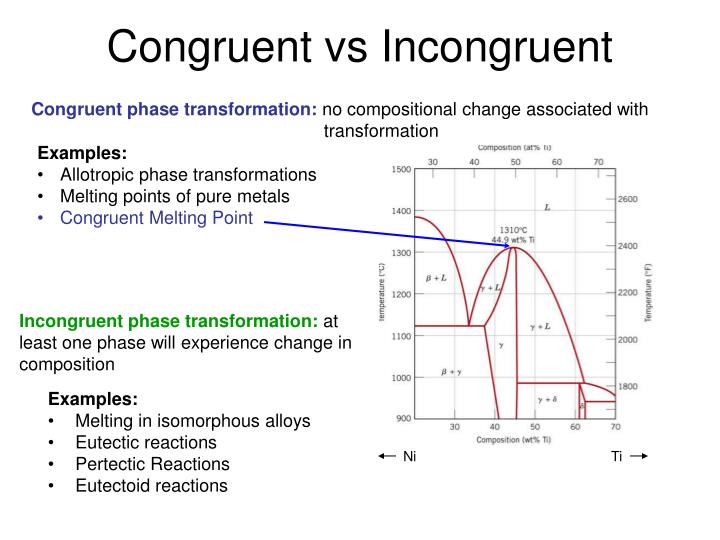
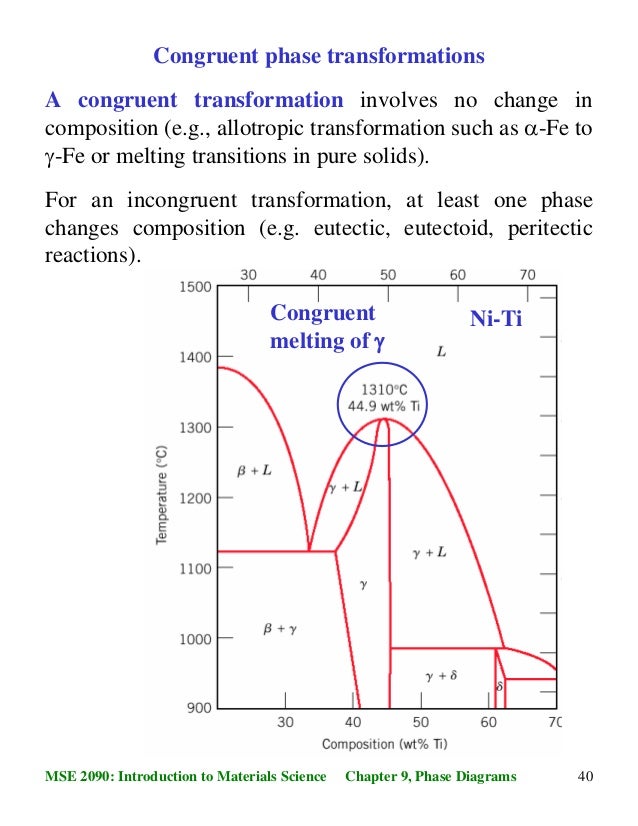
Congruent melting; Incongruent transition; Phase diagram; Congruent melting occurs during melting of a compound when the composition of the liquid that forms is the same as the composition of the solid. It can be contrasted with incongruent melting. This generally happens in two-component systems. To take a general case, let A and B be the two ...
i.e. is the crystal structure of a hypothetical γ″ phase in iron the same as in manganese? Or might one be BCC and the other HCP? New to metallurgy
Congruent melting occurs during melting of a compound when the composition of the liquid that forms is the same as the composition of the solid. It can be contrasted with incongruent melting.This generally happens in two-component systems.To take a general case, let A and B be the two components and AB a stable solid compound formed by their chemical combination.
in the initial solid. These compound are said to possess a congruent melting point with the phase diagram as Figure 19. The general phase diagram of systems forming compounds with congruent melting points. LEGAL NOTICE This document is an excerpt from the book entitled “A Textbook of Physical Chemistry – Volume 1 by
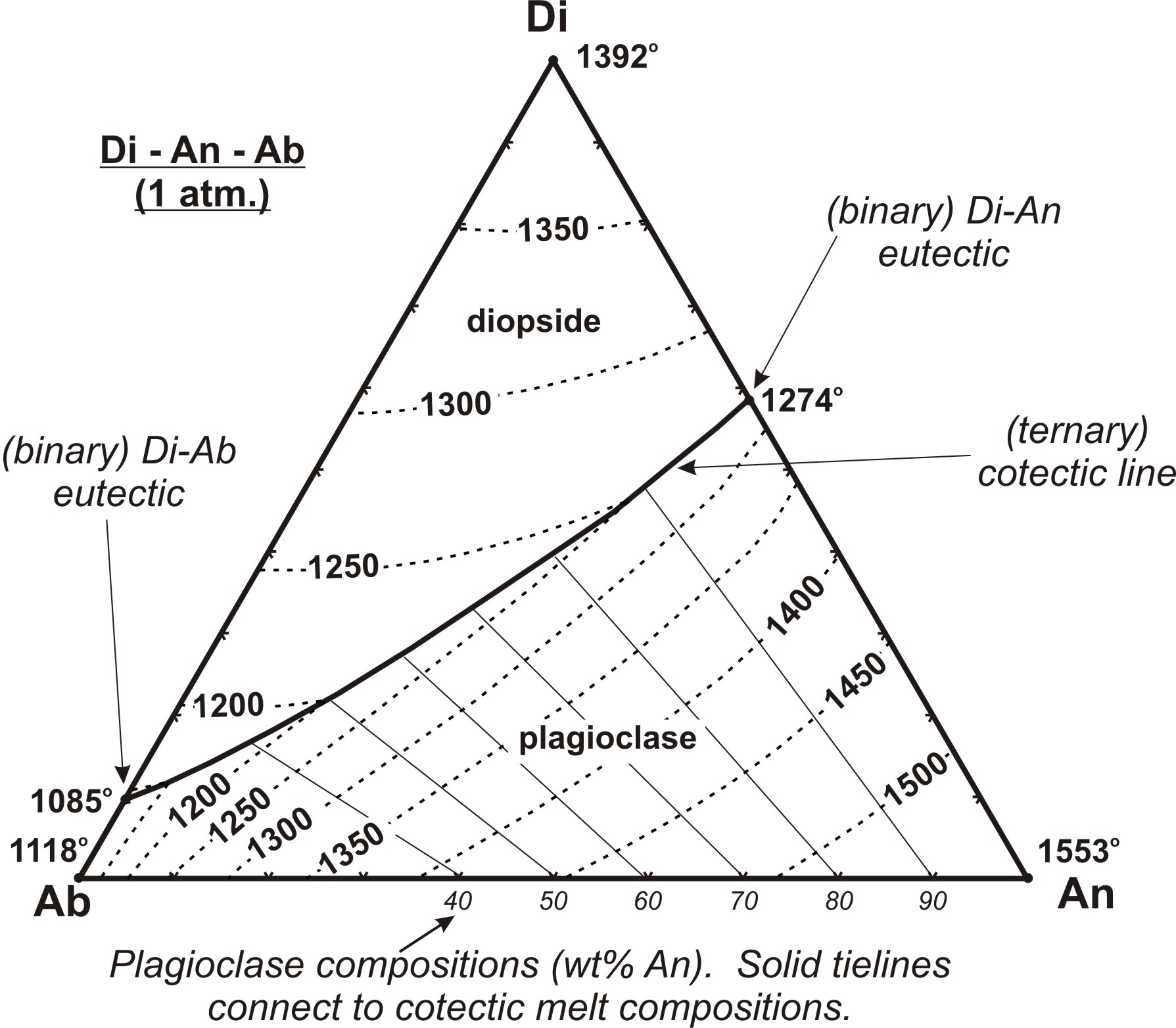
Ternary Phase Diagrams Page 2 of 11 10/14/2003. ... Note that incongruent melting of D continues into the ternary system. Ternary Phase Diagrams Page 6 of 11 10/14/2003. We will consider equilibrium crystallization of compositions P, Q, S, T and X, as all will behave somewhat differently. 1. Crystallization of composition P
Hello all, I am wondering whether anyone could recommend resources with mineral melting points. As many have variable substitutionals, I'd really like the phase diagrams, preferably with the pressure variations.
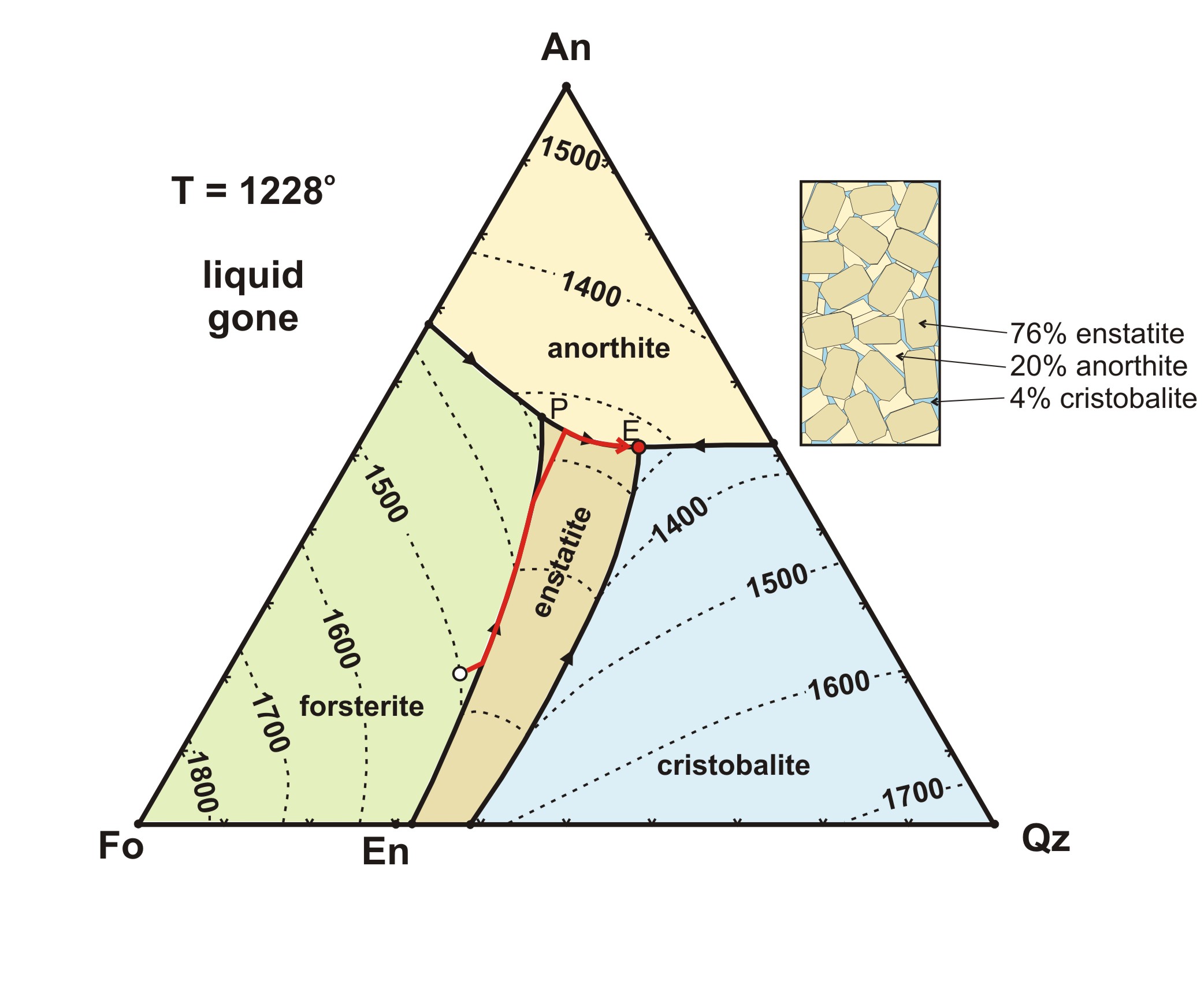
Incongruent melting- melting wherein a phase melts to a liquid with a composition different from the solid and produces a solid of different composition to the original solid. For the case of incongruent melting, we will use the system forsterite (Mg2SiO4) - silica (SiO2), which has an intermediate compound, enstatite (MgSiO3).
1 Na-K system This is a two-component system having incongruent melting point. The melting points of sodium and potassium are 97.8°C and 63.8°C respectively which are shown in the phase diagram in Fig. 2.4. Both elements chemically combine together in the ratio of 2:1 to form a compound Na 2 K.
Bsc Chemistry।। Phase Diagram।। INCONGRUENT MELTING POINT, difference between congruent and incongruent melting point,peritectic or meritectic point, Univers...
BINARY DIAGRAMS - examples. I. Simple 2 component with 2 endmember phases (done above) II. Two component with intermediate compound. A. Two eutectic system (congruent melting) B. Peritectic system (incongruent melting) III. Solid Solution . II. A. Congruent melting - solid phase with composition intermediate between endmembers
Phase relations are commonly described graphically in terms of phase diagrams (see Figure 1). Each point within the diagram indicates a particular combination ...
Binary Peritectic Phase Diagram Equilibrium crystallization of composition X equilibrium crystallization = Fo+En in final rock equilibrium crystallization = En+Cr in final rock Phenocryst composition = 100% Fo (45% of chamber) Incongruent melting
I understand that congruent melting means that the solid and liquid phases have the same composition, and that in incongruent melting, the third intermediate compound decomposes upon melting. However, I am having trouble relating this to the phase diagrams that supposedly depict congruent and incongruent melting. For [congruent](https://slideplayer.com/slide/13310126/80/images/14/Compounds+formation+with+congruent+melting+point..jpg) melting: does the liquid phase then contain all three compoun...
Academia.edu is a platform for academics to share research papers.
**For my first question**, here's the setup. Elemental sodium boils at 883^o C, and iron doesn't even melt until 1538^o C. But on the [binary phase diagram](http://i.imgur.com/PSN1z.png) it shows that sodium stays as a liquid when combined with iron, even though there isn't solubility until about 1394^o C when Fe goes back into BCC ferrite. Can someone explain what is going on with a visual picture? I don't see why the phase diagram says (Fe) rt + L or especially (Fe) ht + L, doesn't that imply ...
The intermediate compound in this diagram (XY2) however is incongruently melting. Incongruent melting is the temperature at which one solid phase transforms to another solid phase and a liquid phase both of different chemical compositions than the original composition. This can be seen in this diagram as XY2 melts to Y and liquid.
The intermediate compound in this diagram (XY 2) however is incongruently melting. Incongruent melting is the temperature at which one solid phase transforms to another solid phase and a liquid phase both of different chemical compositions than the original composition. This can be seen in this diagram as XY 2 melts to Y and liquid.
INCONGRUENT MELTING ! Definitions: ! Liquidus: The line separating the field of all liquid from that of liquid plus crystals. Solidus: The line separating the field of all solid from that of liquid plus crystals. Eutectic point: the point on a phase diagram where the maximum number of allowable phases are in equilibrium. When this point is reached, the temperature
It showed all their developmental products

Melting the Gold and Predicting the Future (Qing dynasty (1644–1911), 1901) // Yu Guiyan (éƒæ¡‚岩 ) Chinese (1859-1930?)
The incongruent melting behavior results in an obvious decrease in crystallinity, leading to the lowest emission intensity. As mentioned above, the preferred orientation of Cs 3 Cu 2 Br 5 samples changes with the calcination temperature, and this result may also account for the difference in emission intensity. Therefore, this work highlights ...
The phase diagram has four maxima corresponding to the formation of four hydrates. Point C (37°C). E(32.5°C) and I(78.5°C) represent the congruent melting point of dodeca, hepta, penta and tetra hydrates respectively. The congruent melting point of a salt hydrate is also known as the dyestectic point. (G) Copper sulphate water vapour system




















0 Response to "41 incongruent melting phase diagram"
Post a Comment