41 atomic orbital diagram for chlorine
Chlorine (Cl) has an atomic mass of 17. ... Electron Configuration, [Ne] 3s2 3p5 ... Relative atomic mass is also known as atomic weight (symbol: Ar). Patents Process for producing porous products Abstract This invention provides a tetrafluoroethylene... shaping, for example, by leaching it out of the shaped article with a solvent or by melting or burning it out.... Therefore, an objective of this invention is the provision of economical processes for producing highly porous...
Perdew, September 28, 2018 (sent for review August 23, 2018; reviewed by Ernest R. Davidson and Mel Levy) Article Figures & SI Info & Metrics PDF Significance Can... molecular orbital (HOMO) (26). Here we propose to define atomic sizes and molecular shapes in terms of the classical turning surface of the exact Kohn–Sham... Search for this keyword Search Advanced Search Home Articles Front Matter News Podcasts Authors Submit atomic...
Atomic orbital diagram for chlorine
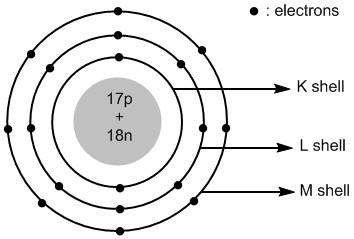
Atomic or orbit structural diagram (g) Formation of Carbon Tetrachloride (CCl 4 ) Carbon 12 6 C (2,4) Carbon needs- four electrons to attain - stable octet. Chlorine 35 17 Cl (2,8,7) Chlorine needs - one electron to attain - stable octet. Nov 6, 2019 ... Get the detailed answer: Create the atomic orbital diagram for chlorine. In a chlorine atom, which subshells contain valence electrons? By convention, each shell is assigned a number and the symbol n—for example, the electron shell closest to the nucleus is called 1n. In order to move between... For instance, if an electron absorbs energy from a photon, it may become excited and move to a higher-energy shell; conversely, when an excited electron drops back...
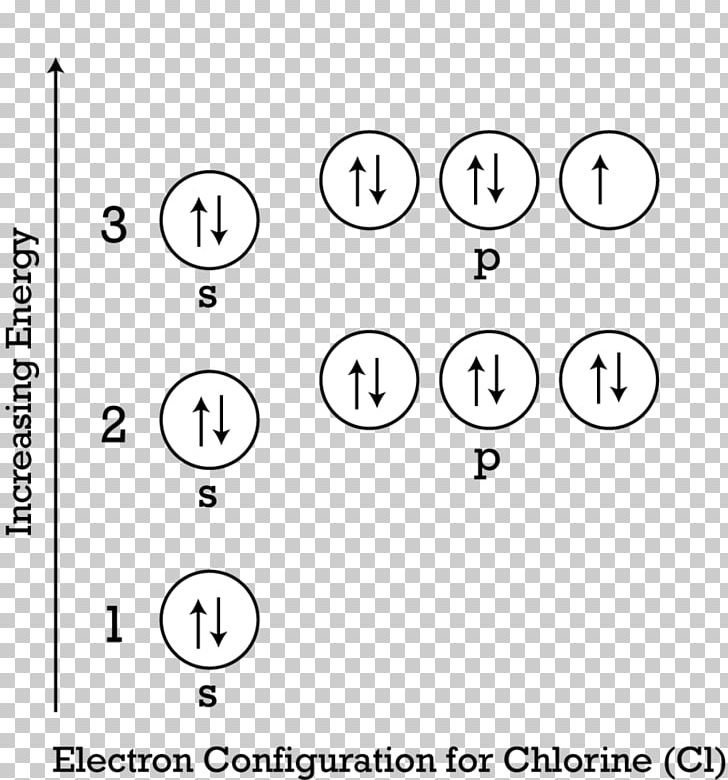
Atomic orbital diagram for chlorine. required and write a partial orbital diagram. PROBLEM: Use partial orbital diagrams to describe how mixing of the atomic orbitals of the central atom(s) leads to hybrid orbitals in each of the following: (a) Methanol, CH. 3. OH (b) Sulfur tetrafluoride, SF. 4 (a) CH. 3. OH. The electron- group arrangement is tetrahedral around both the C and ... Abbreviated orbital diagram of chlorine Description Greenish-yellow, disagreeable gas. Never found in free form in nature. Uses Used in water purification, bleaches, acids and many, many other compounds such as chlorofluorocarbons (CFC). Sources Salt (sodium chloride, NaCl) is its most common compound. The second member of the halogen family, chlorine is represented by Cl and has a total of 17 electrons among which two electrons belong to the K shell, eight to ... In writing the electron configuration for Chlorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chlorine go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two ...
What is the atomic orbital diagram for nitrogen? The remaining three electrons will go in the 2p orbital. Therefore the N electron configuration will be 1s22s22p3. The configuration notation for Nitrogen (N) provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of the Nitrogen atom. View Notes - L37 from CHEM 1A at University of California, Berkeley. Orbital energy diagram for a potassium atom (Z=19) Lecture 37 Breaking the Code: Periodic Table n=2 l=1 ml = +1 ms = +1/2 3s 2p 2s If you paid $37.27 for some denim cloth, what length (in feet) did you buy? A shipping channel is dredged... Here are some old-fashioned units used for measuring volumes: 1 bushel = 1.24 cubic feet (ft3 ) 1 cord = 128... For each of the following atoms or ions, provide the number of protons, neutrons, and electrons: a) 40Ar... Jun 03, 2019 · Molecular Orbital Diagram – Cl2, Br2, I2 3s & 3p and higher atomic orbitals are not so widely separated in energy and allow significant mixing (hybridization) to occur. This mixing causes the inversion of the σσand πmolecular orbitals’ energy. σσσ ππ σ* π* 3,4,5 p 3, 4,5 s σ* σ 3,4,5 s 3,4,5 p Interhalogens Br Br F F Br F F F F.
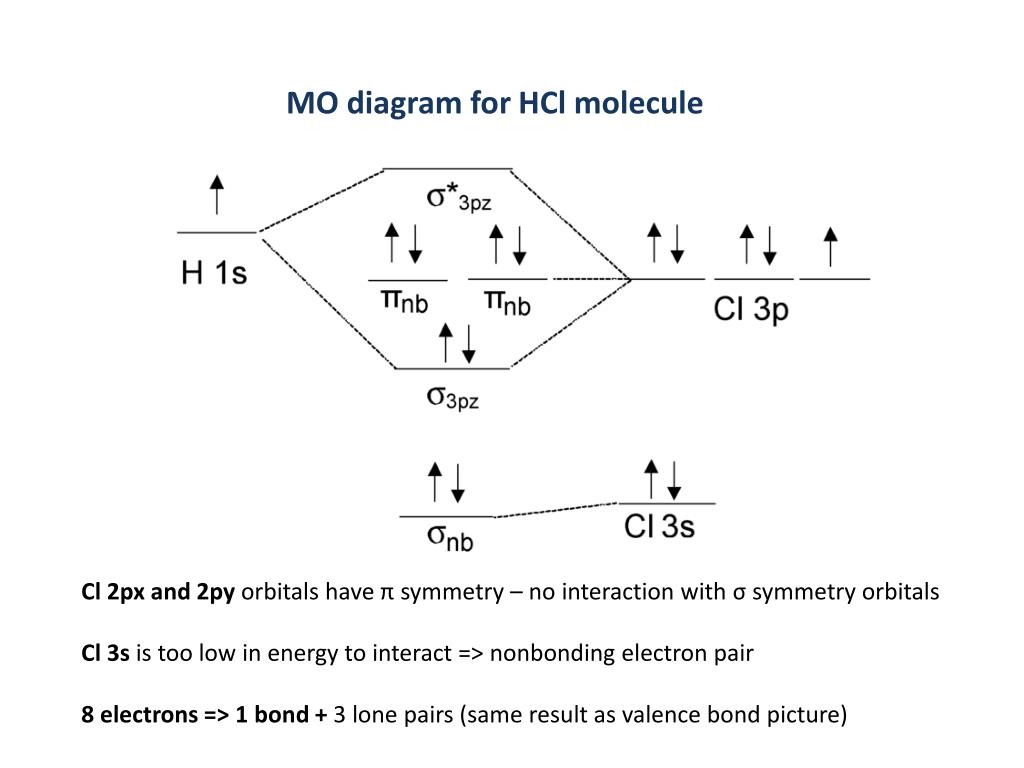
Science. Chemistry. Chemistry questions and answers. Зр Зѕ Energy 2р 2s 1s 1s Answer Bank Fill in the atomic orbital diagram for a chlorine atom. 3p 3 3s Energy. 1 Answer. Truong-Son N. Feb 14, 2016. HCl has no orbital hybridization. Chlorine's 3s is too low in energy to interact with hydrogen's 1s, but chlorine's 3pz can interact with hydrogen's 1s atomic orbital just fine. A good general rule is that being less than about 12 eV apart in energy is required for orbitals to be close enough in energy. For each electron shell atom diagram, the element symbol is listed in the nucleus. The electron shells are shown, moving outward from the nucleus. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The element atomic number and name are listed in the upper left. The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ).
FREE Answer to Create the atomic orbital diagram for chlorine. In a chlorine atom, which subshells contain valence electrons?
03/07/2016 · Finally, the last electron to be added will be placed in the 3p_z orbital, once again having spin-down. Here's a diagram showing the electron configuration of chlorine, with the last electron added highlighted So, the magnetic quantum number, m_l, tells you the specific orbital in which the electron is located. Click to visit.

Health Sciences Center, Stony Brook, New York, Sectional Diagram (c. 1974) // Bertrand Goldberg American, 1913-1997
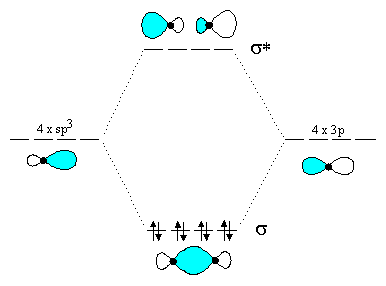
This orbital energy-level diagram shows the sp hybridized orbitals on Be in the linear BeCl 2 molecule. Each of the two sp hybrid orbitals holds one electron and is thus half filled and available for bonding via overlap with a Cl 3p orbital. When atomic orbitals hybridize, the valence electrons occupy the newly created orbitals.
However, complex energy calculations provide essential information (for example the shapes of atomic and molecular orbitals). We do not need to examine the calculations in detail, but we do need to include in our models the information they provide (section 4.8.). Moreover, energy gives us a quantifiable measure of the forces....
However, the diagram above clearly shows that the 4 orbital is filled before the 3 orbital. In other words... For example, sodium (Na), which has a single electron in its outer 3 orbital, can lose that electron to attain the electron configuration of neon. Chlorine, with seven valence electrons, can gain one electron to attain...
Explore the chemical elements through this periodic table ... WebElements uses website cookies to provide the optimise your visit. Learn more I U.Nd.Er.S.Ta.Nd Elements Compounds Shop Printable periodic table The periodic table of the elements The periodic table is an arrangment of the chemical elements ordered by atomic number so that periodic properties of the elements (chemical periodicity) are made clear. Explore the chemical elements through this periodic table Group 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Period 1 1 H 1.008 Hydrogen 2 He 4.0026
Orbital diagram of chlorine 17 ... Generalized energy level diagram for atomic orbitals in an atom with two or more electrons (not for scalar). 5p orbitals fill in immediately after 4d, and immediately before 6s. Filling order is based on observed experimental results, and has been confirmed by theoretical calculations. ...
What are the quantum numbers for chlorine? - The electronic configuration of the chlorine is [Ne]3s23p5 . - Therefore the quantum numbers for chlorine are principal quantum number (n) is 3, azimuthal quantum number (l) = 1 for 3p -orbital, magnetic quantum number (m) is -1, 0 , 1 and spin quantum (s) number is either −12or+12 .
Atomic-scale insights into surface species of electrocatalysts in three dimensions. , 300–305 (2018).Friebel, D. et al. Identification of highly active Fe sites... A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. , 1383–1385 (2011).Grimaud, A. et al. Double perovskites as a...
Cl2 Molecular Orbital Diagram. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in . This means that the σ 1 s molecular orbital has a lower energy than either of the hydrogen 1s atomic orbitals.
Electronic configuration of the Chlorine atom. Valence electrons. Orbital diagram.
The molecular, sp 3 orbitals are arranged in a tetrahedron, with bond angles of 109.5 o. Each of the 1s orbitals of H will overlap with one of these hybrid orbitals to give the predicted tetrahedral geometry and shape of methane, CH 4. Hybridization also changes the energy levels of the orbitals. The 2s orbital of carbon is lower in energy than the 2p orbitals, since it is more penetrating.
Either way, the Chlorine electron configuration will be 1s2 2s2 2p6 3s2 3p5 . Once we have this information we can then place electrons in an ...
Figure 7.5.4. This orbital energy-level diagram shows the sp hybridized orbitals on Be in the linear BeCl 2 molecule. Each of the two sp hybrid orbitals holds one electron and is thus half filled and available for bonding via overlap with a Cl 3p orbital. When atomic orbitals hybridize, the valence electrons occupy the newly created orbitals.
A representation of the atomic spectrum of strontium. The of strontium is 5.03 kJ mol . The of strontium are given below. The following are " " effective nuclear charges,. Follow the hyperlinks for more details and for graphs in various formats.These effective nuclear charges, , are adapted from the following references: E....
The repulsion between the two electrons in the same orbital means that the electron is easier to remove than it would otherwise be. Atomic radius The trend The diagram shows how the atomic radius changes as you go... From sodium to chlorine, the bonding electrons are all in the 3-level, being screened by the electrons in the...
Draw the atomic orbital diagram for chlorine. Learn this topic by watching The Electron Configuration Concept Videos All Chemistry Practice Problems The Electron Configuration Practice Problems Q. Construct the orbital diagram of each atom or ion.TiTi2+Ti4+ Q. Write the corresponding electron configuration for the following pictorial ...
Fluorine(F) is the 9th element in the periodic table and the first element in group-17. The standard atomic mass of fluorine is 18.998403 and its symbol is 'F'. The period of fluorine is 2 and it is a p-block element. The electron configuration of fluorine(F) and the orbital diagram is the main topic of this article.

River City I, Marina City, Chicago, Illinois, Sectional Diagram (N.d.) // Bertrand Goldberg American, 1913–1997
Title Bond Length Caption Figure 7.2 A graph of potential energy versus internuclear distance for the H2... Notes Ionic bonding in NaCl Keywords ionic bond Title Polar Covalent Bond Caption Chlorine is more... the diagram: carbon, nitrogen, and oxygen. Notes Electron-dot structures of polyatomic molecules Keywords polyatomic... two atomic orbitals can be combined in a subtractive... 15 A molecular orbital diagram for the H2 molecule....
(ASED)molecular orbital theory []. Clwas found to readily dissociate over one-fold and two-fold sites [ ]... of atomic chlorine over the Fe(100) surface presented binding chemisorption energies for on-surface and... of chlorine using a plain DFT functional. A thermodynamic stability diagram was constructed over a wide range of...

Atomic Bomb Explosion Before 1952 (c. 1952, printed later) // Harold Eugene Edgerton American, 1903–1990
Jan 26, 2021 — For writing the Chlorine Electron Configuration you first need to check the number of electrons for the Chlorine (Cl) atom (there are 17 ...
Nov 14, 2011 · An orbital is a region of space that an electron can exist in. For the diagram you start with the 1 s orbital and then 2s, 2p, and so on. Each orbital can hold 2 electrons and each arrow ...
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
Figure 4. This orbital energy-level diagram shows the sp hybridized orbitals on Be in the linear BeCl 2 molecule. Each of the two sp hybrid orbitals holds one electron and is thus half filled and available for bonding via overlap with a Cl 3p orbital.
Trends in atomic radius in the Periodic Table The exact pattern you get depends on which measure of atomic radius you use - but the trends are still valid. The following diagram uses metallic radii for metallic elements, covalent radii for elements that form covalent bonds, and van der Waals radii for those (like the noble...
Atomic no. Orbital Diagram of All Elements Diagrams; 1: Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram ...
chemistry questions and answers. Create The Atomic Orbital Diagram For Chlorine. In A Chlorine Atom, Which Subshells Contain ... Question: Create The Atomic Orbital Diagram For Chlorine. In A Chlorine Atom, Which Subshells Contain Valence Electrons?
Answer (1 of 2): Here is a useful MO diagram of HCL found on the internet: The Cl electrons residing up to 3s orbital (1s, 2s, 2px,2py,2pz,3s) are largely stabilized than H electron in 1s orbital and therefore they cannot mix and form bond. The 3p electrons of Cl have comparable energy with the ...
Oct 9, 2019 — The electronic configuration of a ground state chlorine is [Ne]3s23p5 (1s22s22p63s23p5). This means that the 4s, 3d and 4p orbitals shown are ...Orbital 2: none1s2s2px2py2pz3s3px3py3pz4s...
Explanation: Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. We'll need to know how many sublevel is present in each energy level, and in turn, how many electrons each sublevel can accommodate. From the given table, for energy level 1, there's only 1 sublevel, which is called 1s.
Chlorine(Cl) is the 17th element in the periodic table and its symbol is 'Cl'. The electron configuration of chlorine and the orbital diagram is the main topic in this article. Also, valency and valence electrons of chlorine, and compound formation, bond formation have been discussed. Hopefully, after reading this article you will know in ...
By convention, each shell is assigned a number and the symbol n—for example, the electron shell closest to the nucleus is called 1n. In order to move between... For instance, if an electron absorbs energy from a photon, it may become excited and move to a higher-energy shell; conversely, when an excited electron drops back...

Marina City Theater, Chicago, Illinois, Roof and Partial Concrete Frame Development Drawing (1961-1962) // Bertrand Goldberg American, 1913-1997
Nov 6, 2019 ... Get the detailed answer: Create the atomic orbital diagram for chlorine. In a chlorine atom, which subshells contain valence electrons?
Atomic or orbit structural diagram (g) Formation of Carbon Tetrachloride (CCl 4 ) Carbon 12 6 C (2,4) Carbon needs- four electrons to attain - stable octet. Chlorine 35 17 Cl (2,8,7) Chlorine needs - one electron to attain - stable octet.






















0 Response to "41 atomic orbital diagram for chlorine"
Post a Comment