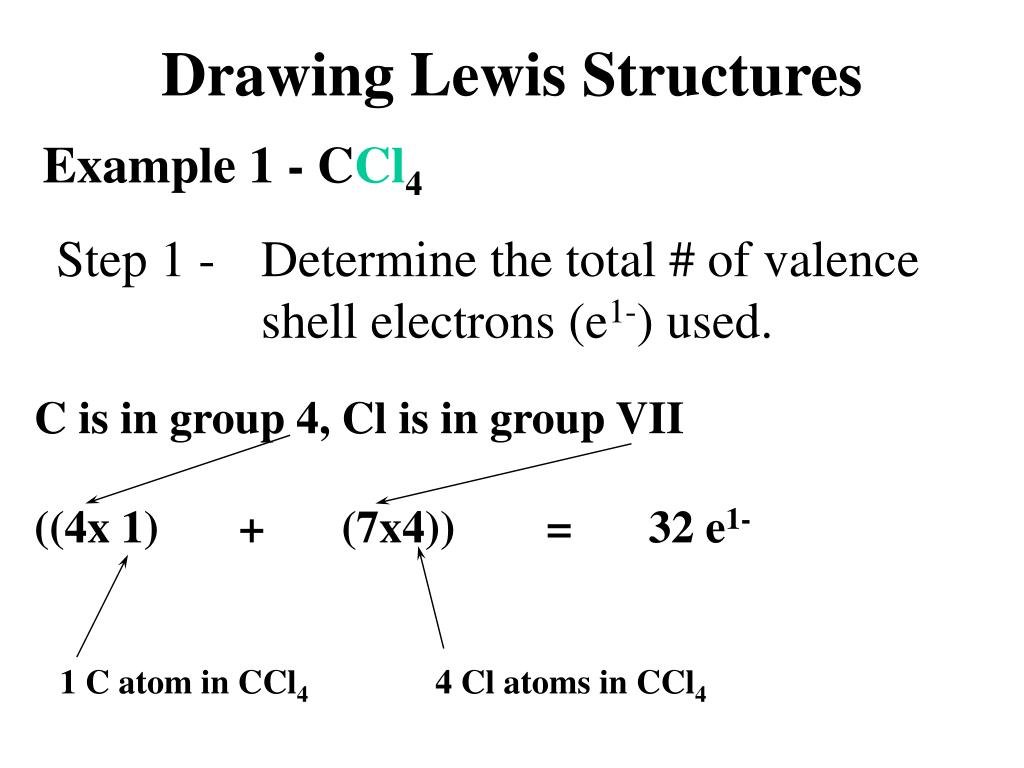
39 lewis dot diagram for ccl4
Jan 28, 2022 · SiO2 has a net dipole moment of zero. It has a linear electron and molecular geometry with a bond angle of 180 degrees and a hybridization of Sp. The Silicon dioxide Lewis structure has a total of 16 valence electrons. In the Lewis dot structure of SiO2, the formal charge is zero. If you have any doubts, please feel free to ask in the comments ... Here's check the molecular geometry and lewis diagram of CCl4. Summary The total valence electron available for the CF4 lewis structure is 32. The hybridization number of CF4 is Sp³ and the steric number is 4. The bond angle of CF4 is 109.5º. CF4 is nonpolar in nature but the bond present in it is polar.
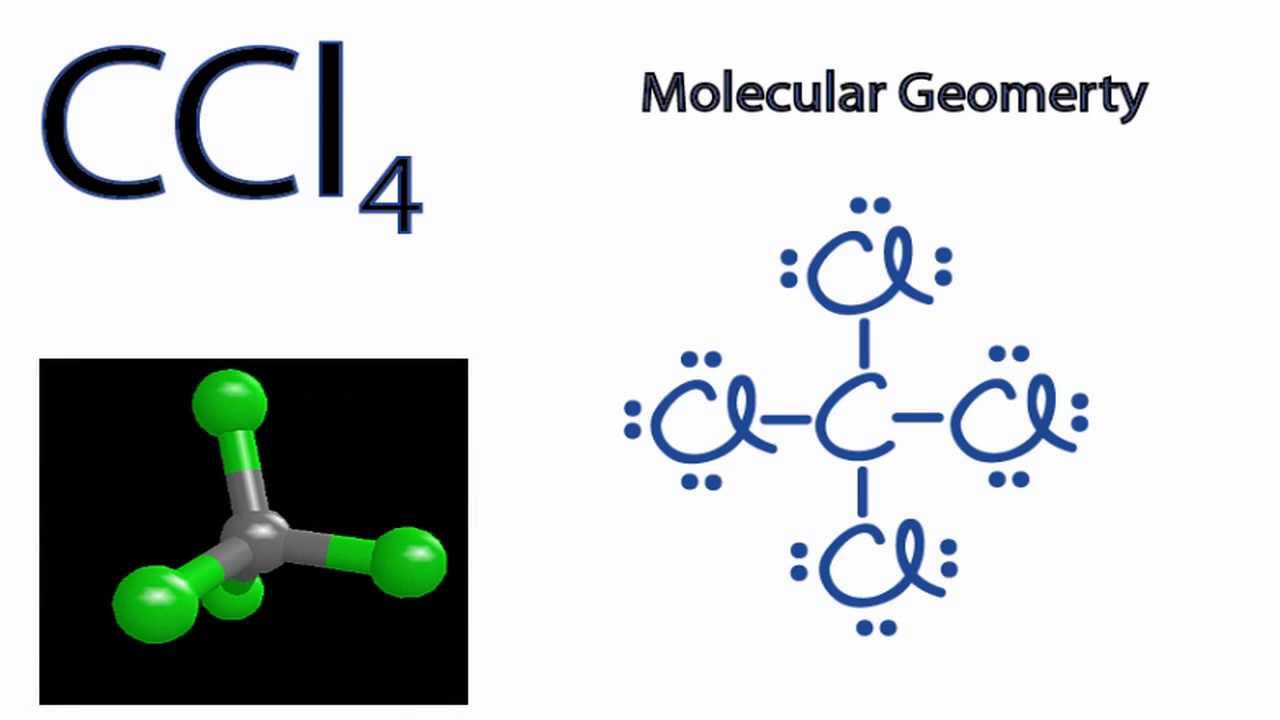
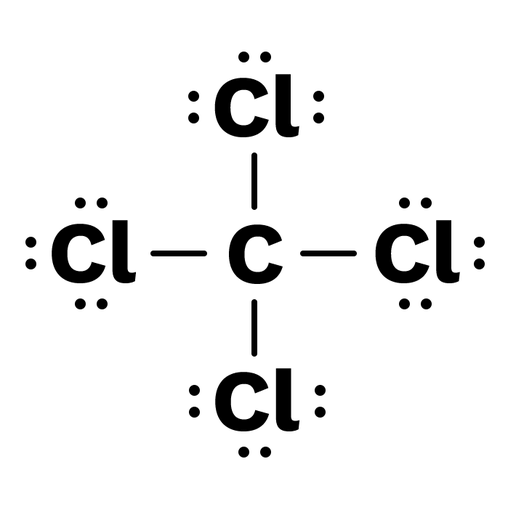
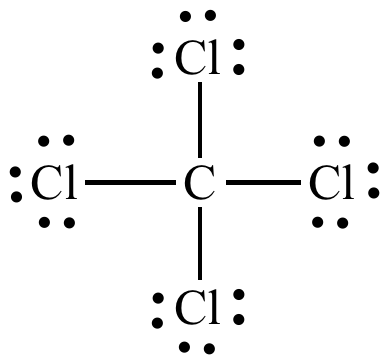
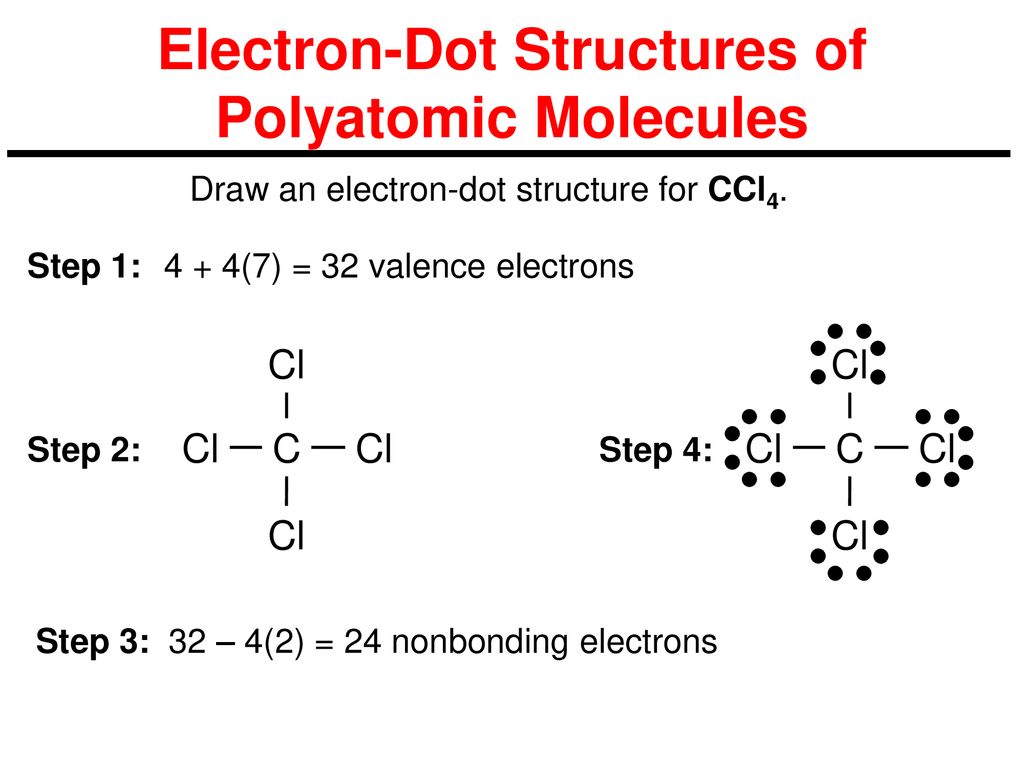
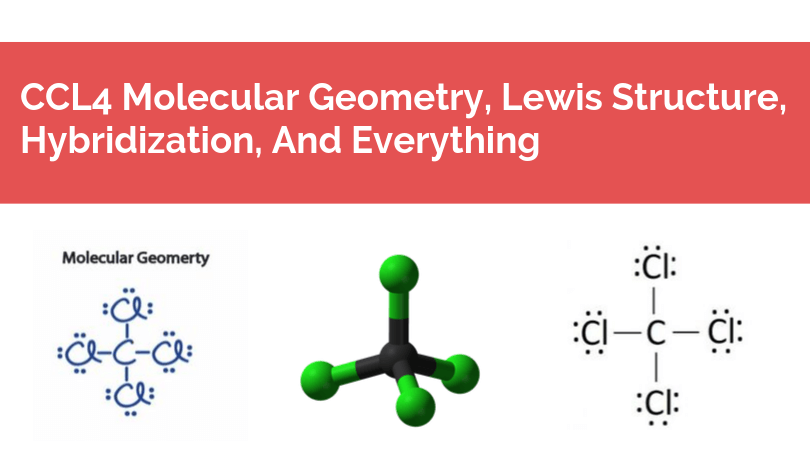
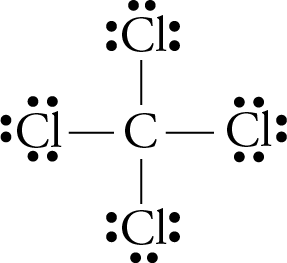
Lewis Dot Structure for CCl4 The Lewis dot structure diagram depicts the placement of electrons in the molecules of any compound. The electrons are represented with the help of circular dots. This diagram displays the bonds formed as well as lone pairs of electrons. Consider the diagram given above.

Lewis dot diagram for ccl4
What is the use of electron dot structure? A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. Answer: Since carbon may have 4 bonds (it has 4 valence electrons), it will bond with the 4 Chloride ions. The dipoles at opposite sides will cancel out (top to bottom & left and right), thus the shape will have 4 equally angled bonds by VSEPR theory and have a tetrahedral structure. Ch4 Dot Structure. Here are a number of highest rated Ch4 Dot Structure pictures upon internet. We identified it from obedient source. Its submitted by government in the best field. We allow this kind of Ch4 Dot Structure graphic could possibly be the most trending topic past we ration it in google help or facebook.
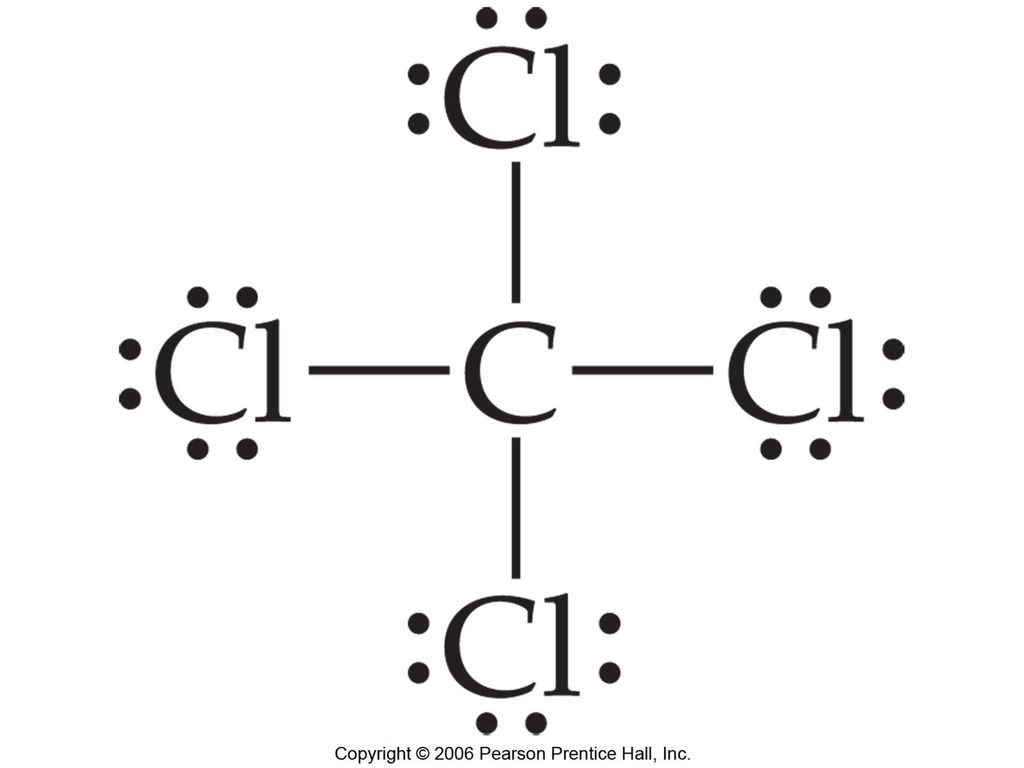
Lewis dot diagram for ccl4. 0 votes. 950 views. asked Dec 11, 2019 in Important Questions by megha00 Expert (44.4k points) What is the Lewis dot structure for CCl4? ClF3 lewis structure contains 3 fluorine atoms at the surrounding position and 1 chlorine atom at the central position. The lewis structure of ClF3 violates the octet as the central atom of it has more than 8 electrons in the outer shell. ClF3 lewis dot structure contains a total of 11 lone pairs(3 on each fluorine atom and 2 on the chlorine atom). Draw a Lewis dot structure for CCl4 including any lone pairs. Best Answer. This is the best answer based on feedback and ratings. The total number of valence electrons in a Lewis diagram is equal to the sum of the valence numbers of the individual elements that compose the compound. Using CCl 4 as our example, the total number of electrons in our diagram is equal to the sum of the valence number of carbon and the valence number for each atom of chlorine.
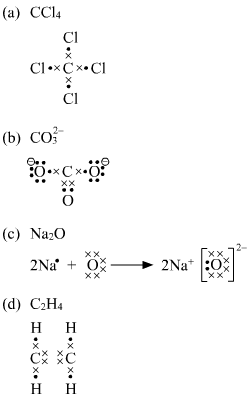
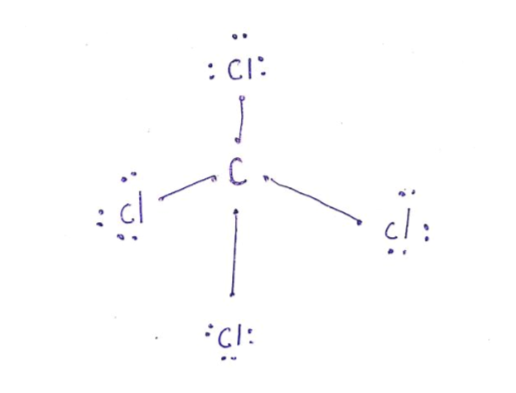
2. Draw the Lewis dot structures for each of the following molecules: a. H 2 S c. SO 3 b. CH 2 Br 2 d. HCN 3. Draw the Lewis dot structure for each of the following polyatomic ions: a. NH 4 + c. PO 4 -3 b. NO 3 - d. CO 3 2- 4. For the following molecules or ions (where the central atom is underlined): i. Draw the Electron dot structure. ii. CCL4 Lewis Structure How to draw the Dot structure for carbon CCL4 is in group 4 or 14 so it has 4. Lewis Point diagram for CCL4. Carbon tetrachloride was synthesized as a by-product in chloroform synthesis. Sometimes, one of the atoms in the molecule does not follow the rule of the batch to arrange pairs of electrons around an atom. We will ... NO DOTS Draw the e- dot diagram for the – ion COMPLETE outer shell Step 3 Enclose both in brackets and show each charge Draw the Lewis Diagrams LiF MgO CaCl2 K2S Drawing molecules using Lewis Dot Structures Symbol represents the KERNEL of the atom (nucleus and inner e-) dots represent valence e- Always remember atoms are trying to complete ... The Lewis dot structure for CCl4 starts with a C in the middle. atom. On the unconnected sides of the Cl atoms, there are two dots, six in total on each atom. What does the Lewis dot structure for...
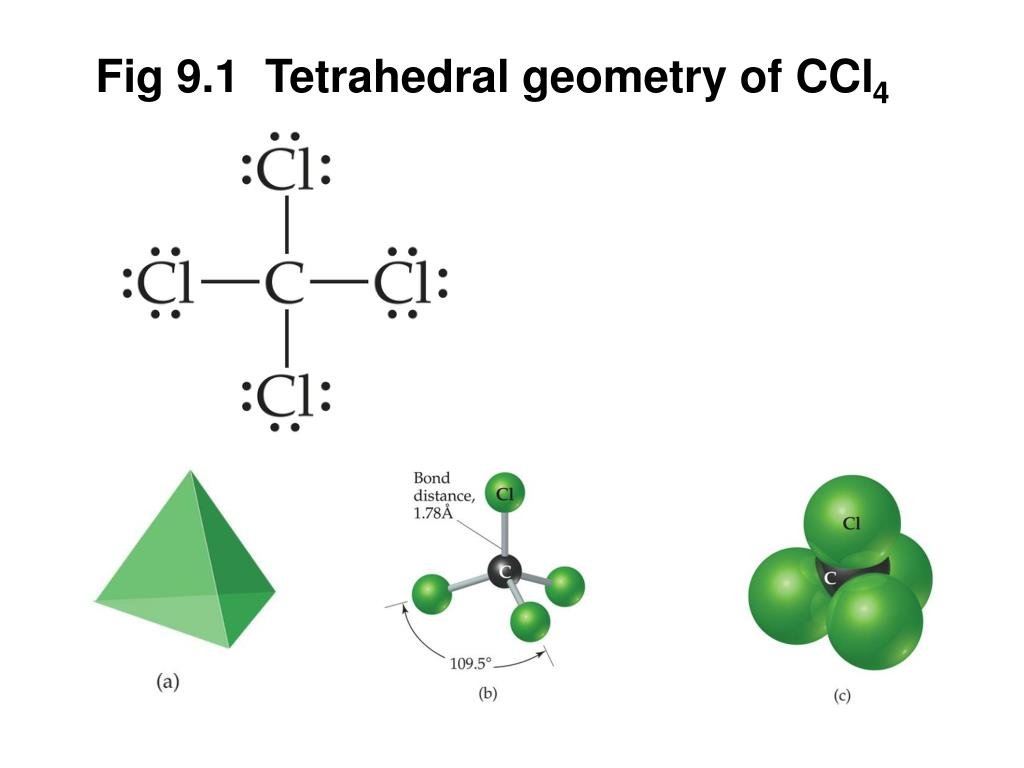
Drawing the Lewis Structure for CCl 4 (Carbon Tetachloride) Viewing Notes: The Lewis structure for CCl 4 is similar to CF 4. Since they are in the same Group on the periodic table they each have the same number of electrons (7) their structures are similar. For the CCl 4 Lewis structure there are a total of 32 valence electrons available. Draw the Lewis dot structure for PF5 and provide the following information. a. number of atoms bonded to the central atom b. number of lone electron … Calculate the total quantity of heat required to convert 25.0 g of liquid CCl4(l) from 35.0°C to gaseous CCl4 76.8°C (the normal boiling point for CCl4)? The specific heat of CCl4(l) is 0.857J (gxC) its heat of fusion is 3.27 kJ/mol and its heat of vaporization is 29.82 kJ/mol The Lewis structure shows that there are four electron regions about the central carbon atom. The VSEPR model states that the electron regions around an atom spread out to make each region is as far from the others as possible. For these clouds in CCl4 to be as far as possible from one another, they will point toward the corners of a tetrahedron.
I quickly take you through how to draw the Lewis Structure of CCl4 (Carbon TetraChloride). I also go over hybridization, shape and bond angle.
Lewis dot structure for Cl2 (Chlorine gas) By looking at the above Cl2 lewis structure, we see both chlorine atoms completed their octet comfortably as both of them have 8 electrons around them. And no need to make any covalent bond in this lewis diagram because we got our stable lewis dot structure for Cl2.
A CCL4 Lewis structure is a diagram that represents the electron configuration of covalently bonded compounds. Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound. A regular atom of carbon has 4 lone electrons in its outer shell.
Step 1: Build a Lewis dot structure for ozone, O3 Total valence electrons (3(6) = 18 Steps 2 and 3: Place one O in the center, and connect the other two O's to it. Drawing a single bond from the central atom to each of the surrounding atoms.
We're being asked to draw a Lewis structure for CCl 4.. To do so, we first need to do the following steps: Step 1: Determine the central atom in this molecule. Step 2: Calculate the total number of valence electrons present. Step 3: Draw the Lewis structure for the molecule.
In a CCl4 Lewis Structure diagram, the carbon atom can be the centre atom. As a result, central carbon in the CCl4 Lewis Structure, with all four Chlorines arranged around the tetrahedral geometry. Step-3: Combining step1 and step2 to get step3 for CCl4 dot structure
What is the Lewis dot structure of CCl4? Lewis Dot Structure for CCl4. The Lewis dot structure diagram depicts the placement of electrons in the molecules of any compound. The electrons are represented with the help of circular dots. This diagram displays the bonds formed as well as lone pairs of electrons.
Enter the email address you signed up with and we'll email you a reset link.
Draw the Lewis dot structure for H2CO and provide the following information. a. number of atoms bonded to the central atom b. number of lone electron …
A CCL4 Lewis structure is a diagram that represents the electron configuration of covalently bonded compounds. Lewis structures are meant to provide a visualization of the atomic structure and the distribution of electrons in a given chemical compound. A regular atom of carbon has 4 lone electrons in its outer shell.
Because of this symmetrical geometry, CCl4 is non-polar. Methane gas has the same structure, making carbon tetrachloride a halomethane. As a solvent, it is well suited to dissolving other non-polar compounds, fats and oils. It can also dissolve iodine. It is somewhat volatile, giving off vapors having a smell characteristic of other chlorinated ...
Ethylene Dichloride is a clear, colorless, oily, synthetic, flammable liquid chlorinated hydrocarbon with a pleasant chloroform-like smell that emits toxic fumes of hydrochloric acid when heated to decomposition. Ethylene dichloride is primarily used to produce vinyl chloride.Inhalation exposure to this substance induces respiratory distress, nausea and vomiting and affects the central …
The Lewis dot structure for CCl4 starts with a C in the middle. atom. On the unconnected sides of the Cl atoms, there are two dots, six in total on each atom. Home Study Guides Science Math and...
The lewis structure for ccl4 is a commonly tested lewis struc. The lewis dot structure for carbon dioxide can be represented like this. A Truncated Version Of The Periodic Table Showing Lewis Dot Structures For The First 20 Elements Chemistry Lessons Chemistry Classroom Middle School Chemistry
The hybridization of CCl4 is sp3. This helps us to understand the geometry of CCl4 which is tetrahedral. The bond angle between the atoms is somewhere around 109 degrees. This is all about the compound CCl4, its Lewis structure, hybridization, molecular geometry, polarity, applications, and MO diagram.
A step-by-step explanation of how to draw the CCl4 Lewis Dot Structure (Carbon tetrachloride). The Lewis structure for CCl4 is a commonly tested Lewis struc...
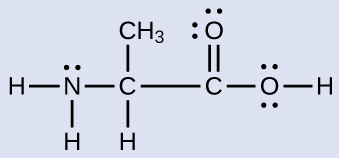
The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3.
For the Lewis structure of CCl4 first, let's calculate the total valence electrons. Carbon has four valence electrons and each Chlorine atom has seven valence electrons. As there are four molecules of Chlorine, we will calculate the number of valence electrons accordingly. = 4 + (4*7) = 4 + 28 = 32 valence electrons
Ch4 Dot Structure. Here are a number of highest rated Ch4 Dot Structure pictures upon internet. We identified it from obedient source. Its submitted by government in the best field. We allow this kind of Ch4 Dot Structure graphic could possibly be the most trending topic past we ration it in google help or facebook.
Answer: Since carbon may have 4 bonds (it has 4 valence electrons), it will bond with the 4 Chloride ions. The dipoles at opposite sides will cancel out (top to bottom & left and right), thus the shape will have 4 equally angled bonds by VSEPR theory and have a tetrahedral structure.
What is the use of electron dot structure? A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms.


















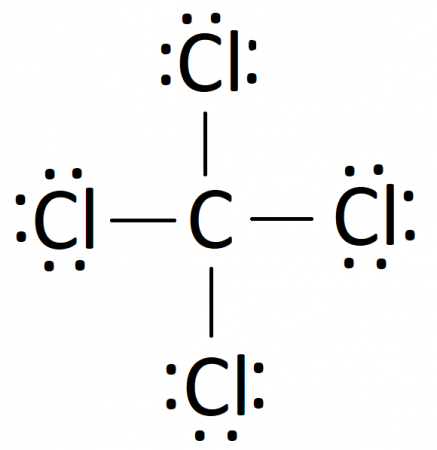

0 Response to "39 lewis dot diagram for ccl4"
Post a Comment