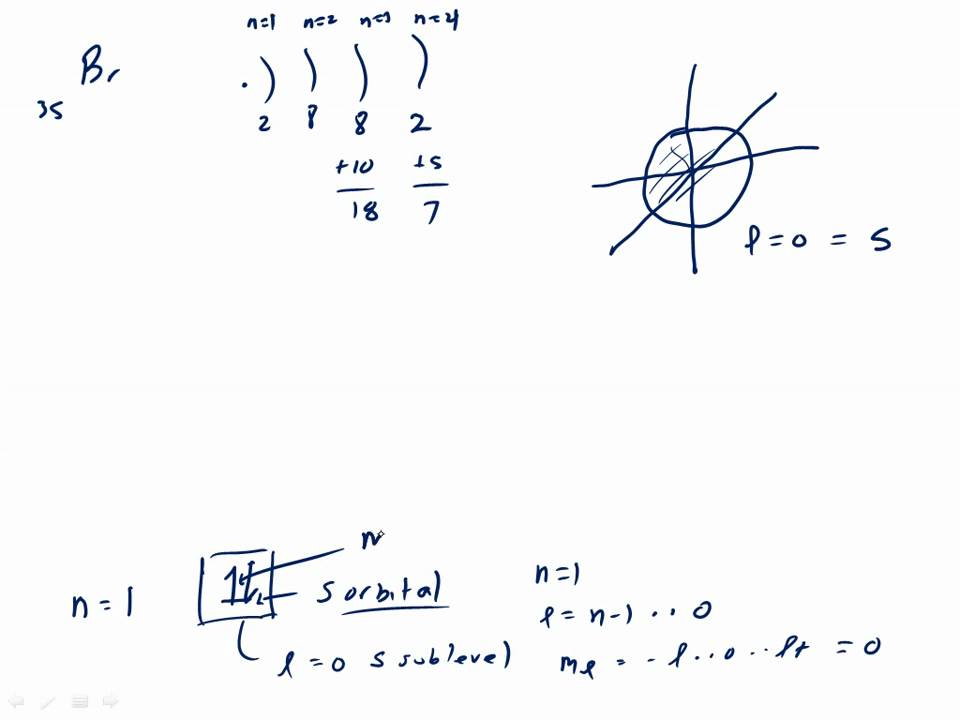
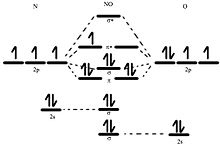
37 orbital filling diagram for bromine
1 answerWe will first determine the number of electrons that a neutral bromine (Br) atom has by referring to its atomic number on a periodic table. Bromine's... ... the electron configuration and give the orbital diagram of a bromine (Br) ... the electron configuration in the order in which the subshells are filled.
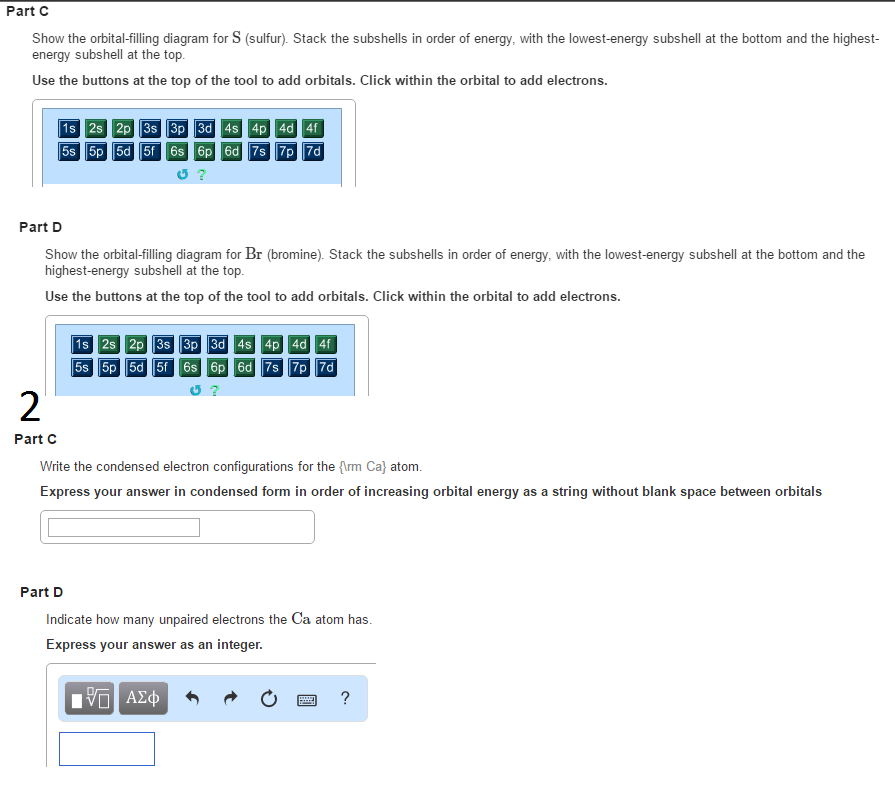
... how to write electron configurations and draw orbital diagrams ... filled according to their energy level, but a more convenient method is to use the.2 pages

Orbital filling diagram for bromine
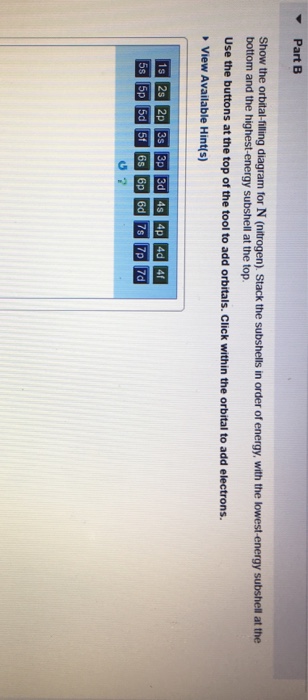
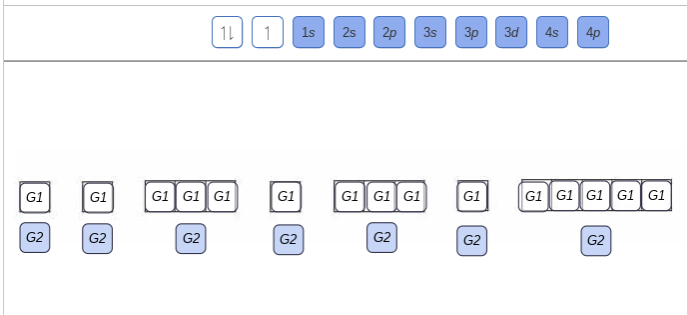
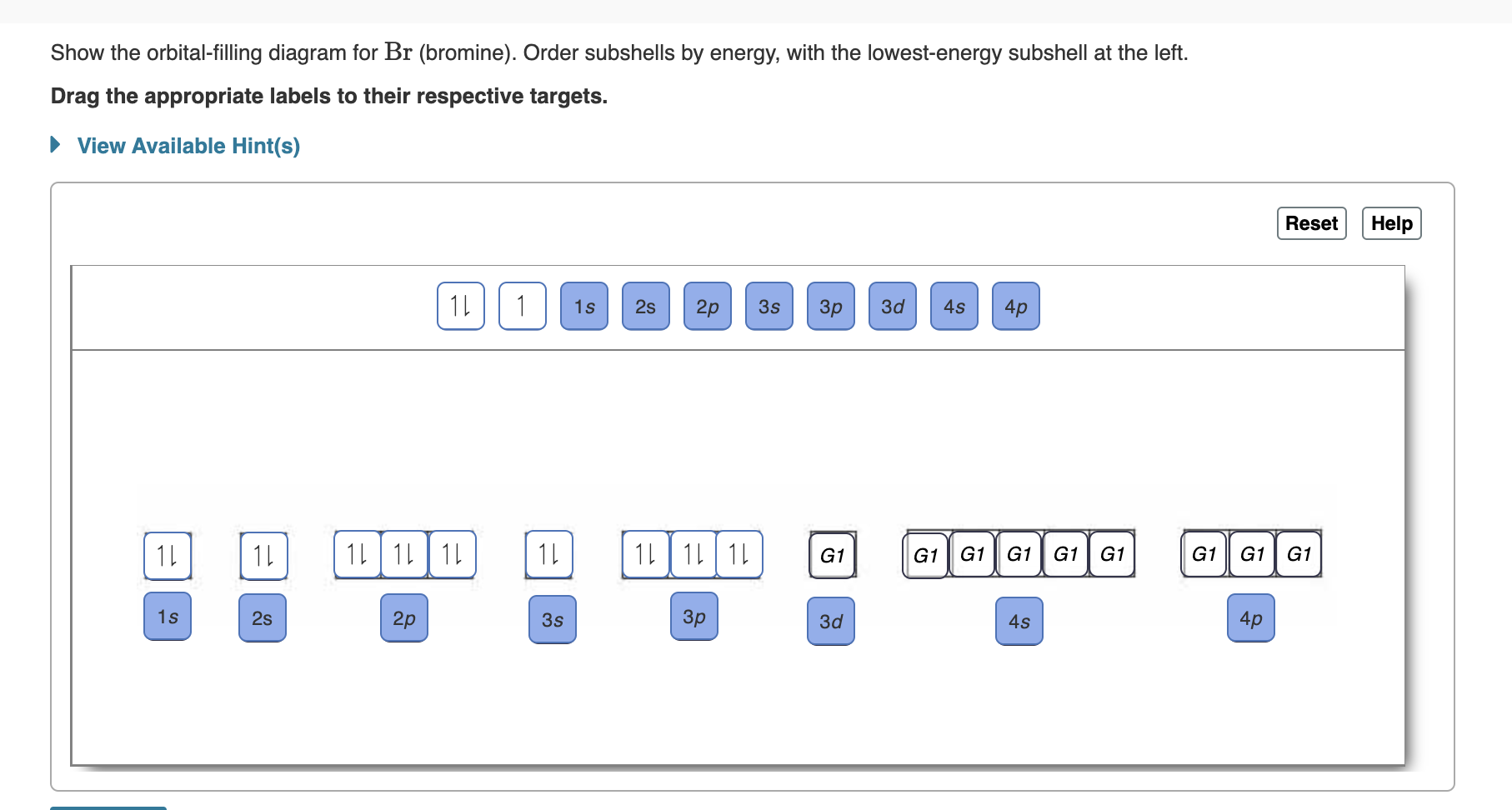
May 9, 2021 — Bromine Orbital Diagram. You can get the idea of an atomic number of the details with the help of a periodic table if you want to know more ... Feb 1, 2021 — Bromine will have 35 arrows placed in the orbital-filling diagram as in figure 13 because it has 35 electrons. The order of the filling is ... Problem: Part D. Show the orbital-filling diagram for Br (bromine). Order subshells by energy, with the lowest-energy subshell at the left.1 answer · Top answer: Recall that for a neutral element, Atomic number = # of protons = # of electrons. The atomic number of Br is 35 and since it’s a neutral element, this ...
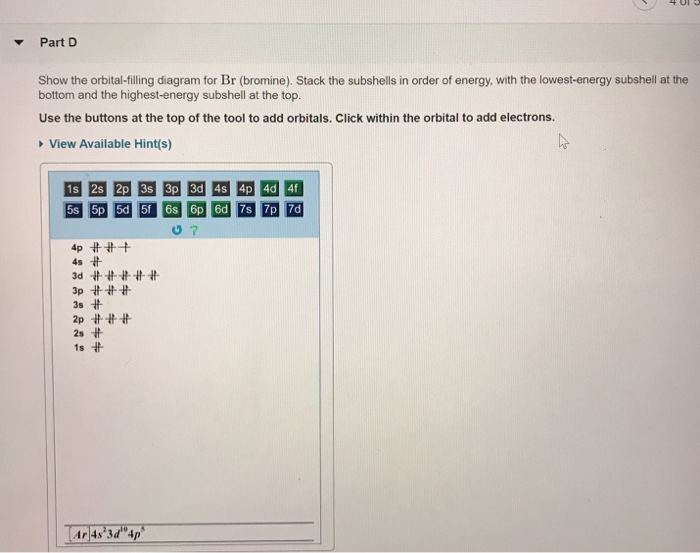
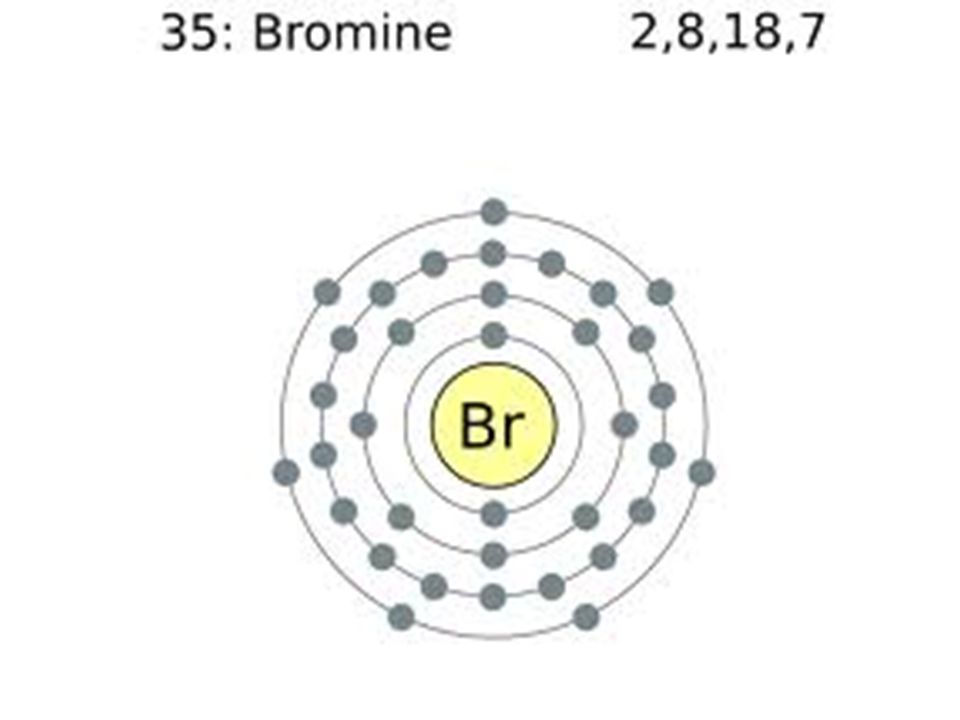
Orbital filling diagram for bromine. Oxidation States, ±1,+5. Electrons Per Shell, 2 8 18 7. Electron Configuration, [Ar] 4s2 3d10 4p5. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5. Orbital Diagram. Problem: Part D. Show the orbital-filling diagram for Br (bromine). Order subshells by energy, with the lowest-energy subshell at the left.1 answer · Top answer: Recall that for a neutral element, Atomic number = # of protons = # of electrons. The atomic number of Br is 35 and since it’s a neutral element, this ... Feb 1, 2021 — Bromine will have 35 arrows placed in the orbital-filling diagram as in figure 13 because it has 35 electrons. The order of the filling is ... May 9, 2021 — Bromine Orbital Diagram. You can get the idea of an atomic number of the details with the help of a periodic table if you want to know more ...












/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)







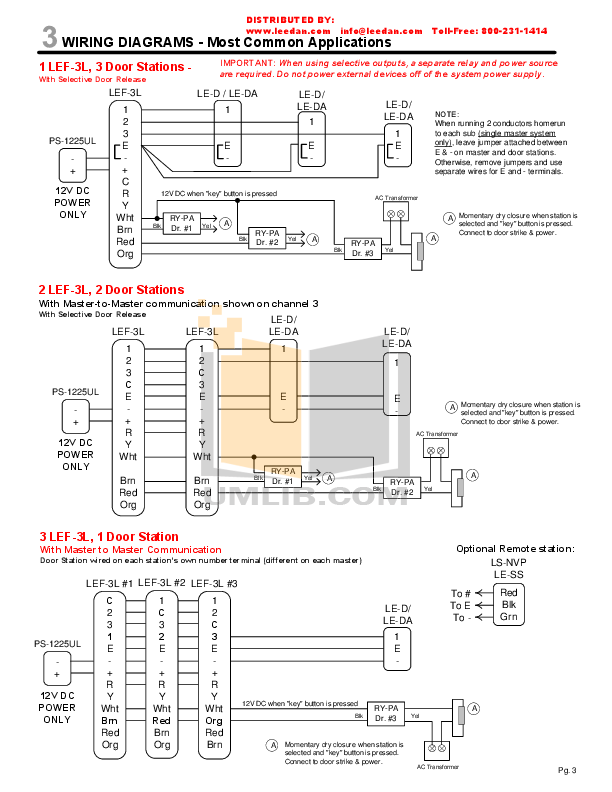

0 Response to "37 orbital filling diagram for bromine"
Post a Comment