37 o2 2- molecular orbital diagram
are combined. The molecular orbital diagram for an O2molecule would therefore ignore the 1selectrons on both oxygen atoms and concentrate on the interactions between the 2sand 2pvalence orbitals. Molecular Orbitals of the Second Energy Level The 2sorbitals on one atom combine with the 2sorbitals on another to form a 2sbonding and a 2s* For Free best Chemistry Notes, visit here👉 https://digitalkemistry.wordpress.com/For more informative Chemistry Lessons !!Subscribe 👉 https://www.youtube...
the atom stlcc edu, worksheet energy levels sublevels orbitals name of, molecular orbital theory purdue university, electron filling sequence article about electron filling, 6 4 electronic structure of atoms electron configurations, constructing the o2 molecular orbital energy level diagram, diagrams name chem worksheet 5 5, ch301 fall 2009 worksheet 3 answer key on electronic, quiz amp ...
O2 2- molecular orbital diagram
28 Oct 2014 — Ans: The stabilities of these can be best explained using Molecular orbital theory. ... Atomic orbitals of oxygen combine to form molecular ... This is the peroxide ion, O2(2-), so you KNOW it's going to be stable.It has a bond order of 1, which also makes sense. Draw the Lewis diagram of hydrogen pe... 3 Mar 2021 — Molecular#Orbital#Diagram #Oxygen#Molecule#MOdiagram #Chemistry #Class11 #NEET #JEE #MDCAT #ECAT #ChemicalBonding This video is a small ...
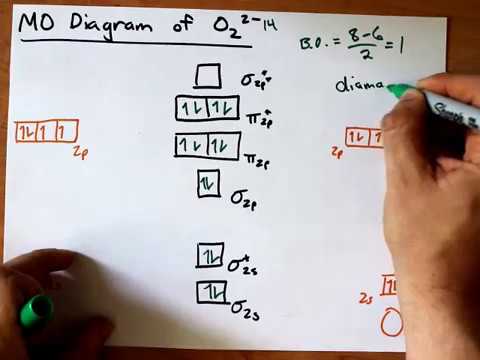
O2 2- molecular orbital diagram. 2. is as follows : O . 2:KK(σ . 2s) 2 (σ . 2s. ∙) 2 (π . 2p. x) 2 =(π . 2p. y) 2 (π . 2p. x) 1 =(π . 2p. y. ∙) 1. The molecular orbital energy level diagram of oxygen molecule is given as follows : Bond order . 2. N . b. −N . a = 2. 8−4 =2. Thus, oxygen molecule has two bonds. i.e., one is bond and one p bond. The last two ... Electronic structure of oxygen atom is Leaving out the 4 electrons in the 1s orbitals of two oxygen atoms constituting the molecule (represented as KK), the molecular orbital energy diagram for remaining 12 electrons of oxygen as molecule is shown:(i) Electronic configuration:(ii) Bond order: Here Nb = 8; Na = 4The two oxygen atoms in a molecule of oxygen are united through two covalent bonds (i.e. a double bond).(iii) Paramagnetic character : Since a molecule of oxygen has two unpaired ... Apr 25, 2018 · You'll need the molecular orbital (MO) diagram of "O"_2. Begin with the atomic orbitals. Oxygen atom has 2s and 2p valence orbitals and 6 valence electrons: Each oxygen contributes 6, so we distribute 12 valence electrons into the molecule to get "O"_2. Two 2s orbitals combine to give a sigma_(2s) bonding and sigma_(2s)^"*" antibonding MO. The electron configuration is written as (s 1s) 2. We can make up a molecular orbital diagram for the He 2 molecule (Fig-ure 3.36). Two electrons decrease in energy on formation of the molecular orbitals, while two electrons increase in energy by the same quantity. Thus, there is no net decrease in energy by bond formation.
To obtain the molecular orbital energy-level diagram for (ce {O2}), we need to place 12 valence electrons (6 from each O atom) in the energy-level diagram shown in Figure (PageIndex {1}). We again fill the orbitals according to Hund’s rules and the Pauli principle, beginning with the orbital that is lowest in energy. Molecular Orbital Diagram for Oxygen Gas (O2).Fill from the bottom up, with 12 electrons total.Bonding Order is 2, and it is Paramagnetic.sigma2s(2),sigma2s*... Draw molecular orbital diagrams for O2-, O22-, and O2. Which has the highest bond order? Which would be paramagnetic, and which would be diamagnetic? Can you draw good dot structures that correspond to each of these ions or molecules? Draw a molecular orbital diagram lor Arz*. This ion has been observed in the gas phase. molecular orbital diagram of O2-Electronic configuration of O2-Advertisement Advertisement itemderby itemderby Explanation: In a molecule, there are total 16 electrons. The molecular orbital configuration of molecule is as follows. The formula for bond order is as follows.
Molecular orbital (MO) theory explains the construction of molecular orbital diagram on the basis of following main points . 1.Formation of MOs: Atomic orbitals(AOs) linearly combine with each other to form equal number of molecular orbitals (MOs). 2.Energy of MOs: Half of the molecular orbitals (MOs) having energy lower than the atomic orbitals are called… As it can be seen from the MOT of O 2 , The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [N b − N a ] / 2 = [1 0 − 6] / 2 = 2. Therefore there is a double bond present as O = O. Dec 11, 2021 · The Molecular orbital diagram for O2 O 2 is like this: As you can see the oxygen molecule has two unpaired electrons in the lower π π * ant-bonding states. For O2+2 O 22 + basically remove the two unpaired electrons in the π π * anti-bonding states, as they are the most easily removed. In this video, you will study about Molecular Orbital diagram of O2, O2+, O2(2-). We will also calculate the Bond order in each case and also the magnetic be...
Transcribed image text: Complete this valence molecular-orbital diagram for oxygen, O2. Click the blue boxes to add electrons as needed.
Definition of atomic orbital diagram for oxygen: An orbital is the region of space around the nucleus within which the probability finding an electron of given energy is maximum. The diagram of this region gives the diagram of the orbital. The plot of angular wave function or square of angular wave functions give us the diagram of orbitals.
As bond dissociation energies are directly proportional to the bond order, therefore, the dissociation energies of these molecular species are in the order: O 2 + > O 2 > O 2 - > O 2 2-As bond length is inversely proportional to bond order, therefore, bond length will be in the order: O 2 2-> O 2 - > O 2 > O 2 + 18) Fluorine molecule (F 2)
Orbital diagram, after Barrett (2002), showing the participating atomic orbitals from each oxygen atom, the molecular orbitals that result from their overlap, and the aufbau filling of the orbitals with the 12 electrons, 6 from each O atom, beginning from the lowest-energy orbitals, and resulting in covalent double-bond character from filled ...
It is sigma2s(2)sigma2s*(2)sigma2p(2)pi2p(4)pi2p*(4)Bond order 1. It is stable. In fact, it's the perioxide ion.Check me out: http://www.chemistnate.com
Draw molecular orbital diagram of O2 or N2 with magnetic behavior and bond order. As it can be seen from the MOT of O2, The electrons in the highest occupied molecular orbital are unpaired therefore it is paramagnetic in nature. Also, the bond order can be calculated as [Nb−Na]/2=[10−6]/2=2.
O2. Therefore, NF is predicted to be paramagnetic with a bond order of 2. The populations of the bonding (8 electrons) and antibonding (4 electrons) molecular orbitals in the diagram suggest a double bond. c. The 2s, 2s *, 2p, and 2p * orbitals exhibit C v symmetry, with the NF bond axis the infinite-fold rotation axis. The 2p and 2p *
Here is the full molecular orbital diagram for O 2. Now we add the 12 electrons, 6 from each oxygen atom. There is one electron each in the 2 pi antibonding orbitals. This means that there is only 1 net pi bond. Also, the molecules has 2 unpaired electrons and therefore has magnetic properties. Molecules with half-filled orbitals are called ...
The total number of electrons present in the ${N_2}$ molecule is 14. Number of electrons in bonding orbitals : 8 Number of electrons in antibonding orbitals : 2 So, the formula to find bond order is Bond order = $\dfrac{1}{2}$ (Number of electrons in BMO) - (Number of electrons in ABMO) Bond order = $\dfrac{1}{2}$ (8) - (2)
Remember: When two oxygen atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals. They are flipped compare...
The Molecular orbital diagram for O2 O 2 is like this: As you can see the oxygen molecule has two unpaired electrons in the lower π π * ant-bonding states. For O2+2 O 22 + basically remove the two unpaired electrons in the π π * anti-bonding states, as they are the most easily removed. 26.2K views View upvotes Related Answer Kusum Khatri , Lecturer
O 2 2- a molecule, there are 18 electrons. The MOT diagram is as shown below, So, the molecular orbital configuration is as follows: σ 1 s 2, σ * 1 s 2 , σ 2 s 2 , σ * 2 s 2 , σ 2 p 2 z, 2p x 2 π = 2p y 2 π , 2p x 2 π * =2p y 2 π * There are 10 bonding electrons and 8 nonbonding electrons. Therefore, bond order = 1 2 [ Bonding-antibonding]
3 Mar 2021 — Molecular#Orbital#Diagram #Oxygen#Molecule#MOdiagram #Chemistry #Class11 #NEET #JEE #MDCAT #ECAT #ChemicalBonding This video is a small ...
This is the peroxide ion, O2(2-), so you KNOW it's going to be stable.It has a bond order of 1, which also makes sense. Draw the Lewis diagram of hydrogen pe...
28 Oct 2014 — Ans: The stabilities of these can be best explained using Molecular orbital theory. ... Atomic orbitals of oxygen combine to form molecular ...

0 Response to "37 o2 2- molecular orbital diagram"
Post a Comment