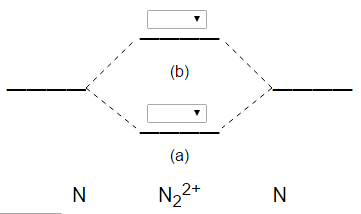
42 complete the mo energy diagram for the n2+ ion.
Since the bond order of Li 2 is higher than Li 2 + and Li 2 - . Therefore ,Li2 has stronger single covalent bonding . Thus lithium molecule (Li2) is more stable. Li 2 + is more stable as compared to Li 2 - :. Li 2 + and Li 2 - ions have the same bond order ( 0.5) .. But Li 2 - has more electrons in higher energy antibonding molecular orbital as compared to Li 2 + .
FREE Answer to Complete the MO energy diagram for the N2+ ion by dragging theelectrons , , and in...1 answer · Top answer: Nitrogen(N) : Atomic no. 7. Electronic configuration of 1s2 2s2 2p3. As single nitrogen contain 7 elcetrons therfore N2 molecule will have ...
An ionic bond is a type of chemical bond in which the atoms have different electronegativity values from each other. For example, sodium (Na) and chlorine (Cl) form an ionic bond to make NaCl (table salt). However, in a covalent bond, the atoms are bound to share electrons. For example, if we talk about water ( H2O), it is a polar covalent bond.
Complete the mo energy diagram for the n2+ ion.
Electron Configuration For Vanadium Ion. The atomic number of Vanadium is 23 and the unique thing about the atomic number is that it is different and no two atomic numbers can be the same. For a student, it is very important to know the basics of chemistry, and knowing terms such as electronic configuration and atomic number is a must.
CO2 Molecular Orbital (MO) Diagram. The molecular orbital diagram of CO2 is as below. A molecular orbital diagram of any compound gives us an idea about the bonding of the orbitals. It also helps us to find the bond order, bond length, bond strength of the molecule. In the diagram, the left-hand side consists of the atomic orbitals of carbon.
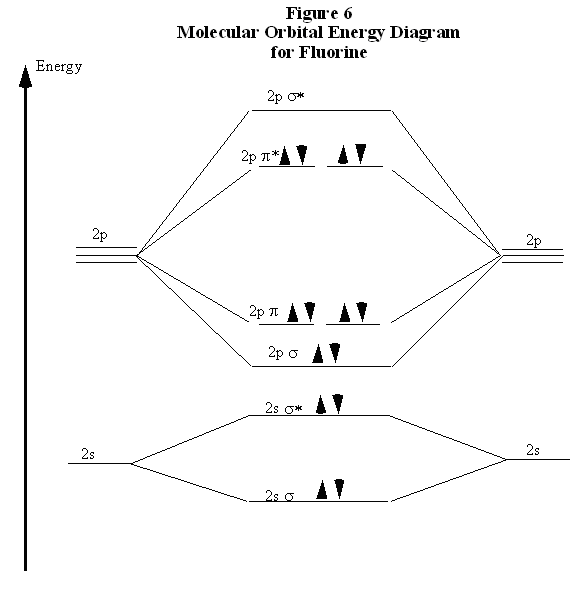
Figure 9.8.1: Molecular Orbital Energy-Level Diagrams for Homonuclear Diatomic Molecules. (a) For F 2, with 14 valence electrons (7 from each F atom), all of the energy levels except the highest, σ ⋆ 2pz are filled. This diagram shows 8 electrons in bonding orbitals and 6 in antibonding orbitals, resulting in a bond order of 1.
Complete the mo energy diagram for the n2+ ion..
a) Gibbs free energy diagrams on the 1T-MoS 2 surface along the respective optimum NRR pathway and the 2H-MoS 2 surface. The PDS for each reaction pathway is marked. b) HER Gibbs free energy diagrams on the 1T-MoS 2 and 2H-MoS 2 surface. The asterisk * in a) and b) denotes the atom in the adsorbate which is bound to the 1T-MoS 2 and 2H-MoS 2 ...
8. Draw a Frost's circle for each ion or molecule, showing the energy levels of the molecular orbitals Indicate the occupancy of each orbital. Assuming planarity, use the MO's to determine if each lon would be aromatic or antiaromatic. 9. Draw an orbital diagram of the system for each molecule, making clear the orientation of each lone pair.
Solut ion. We draw a molecular orbital energy diagram similar to that shown in Figure 11. Molecular Orbital Diagram s simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagram s is one of the trickier concepts in chemistry. The first major step is understanding ...
Chemical equation or formula questions. Updated 29 Jul 2021. You can enter answers to these chemistry questions on a computer, tablet, or smartphone. To practice entering chemical expression answers. When available, open the Introduction to Mastering assignment and select the the Introduction to Chemical Answers item.
34 Molecular Orbital Diagram For N2; 21 Poulan 2075 Chainsaw Fuel Line Diagram; 24 2003 Chevy Impala Radio Wiring Diagram; 35 Wayne Pump Parts Diagram; 34 John Deere D140 Drive Belt Diagram; 25 Buddhism And Hinduism Venn Diagram; 24 Jablonski Energy Diagram; 23 Craftsman Leaf Blower Fuel Line Diagram; 35 Rosary Diagram Worksheet; 29 Chrysler ...
Tim Soalkimia.com telah merangkum contoh soal Laju Reaksi untuk anda latihan dirumah ataupun persiapan ulangan harian yang berjumlah 60 butir dari berbagai penerbit buku seperti Erlangga, Unggul Sudarmo Kurikulum 2013 semoga kamu dapat memahami materi terkait laju reaksi.. Simak Juga Materi : Laju Reaksi 1- 15 Soal Laju Reaksi dan Pembahasan. 1. Kenaikan suhu akan mempercepat laju reaksi ...
Are you looking for a blog post to help you with understanding the PCl3 Lewis Structure and its molecular geometry in detail? If yes then check out this article of PCl3 Molecule.
Molecular Orbital Diagram for Nitrogen Gas ( 1 ion) (N2( )). Fill from the bottom up, with 9 valence electrons total. Comparing and Contrasting the Molecular Orbital Diagram s of N2- and N2+.In this video lecture Molecular Orbital Energy Level Diagram s for N2, N2 , N2-, N2 2- C2 and B2 are.... Fig. No. 1 Molecular Orbital Diagram for H2 molecule. Comparison of N2 and N2+ ion N 2 + ion is for ...
Molybdenum is a chemical element with the symbol Mo and atomic number 42. The name is from Neo-Latin molybdaenum, which is based on Ancient Greek Μόλυβδος molybdos, meaning lead, since its ores were confused with lead ores. Molybdenum minerals have been known throughout history, but the element was discovered (in the sense of differentiating it as a new entity from the mineral salts of ...
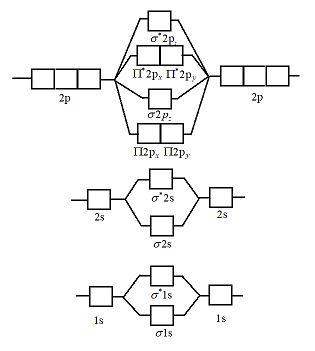
Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al ...
Electron Configuration For Nitrogen Ion. The atomic number of nitrogen is 7, the element nitrogen was discovered by a Scottish physician, Danial Rutherford. The year the element was discovered in the year 1772, through our article you will come to know about certain new things about the element Nitrogen.
Complete step by step answer: Molecular orbital diagram explains the chemical bonding in the molecules in terms of molecular orbital theory. In a molecular ...
The electron arrangement of an atom at its lowest possible energy state is known as the ground state electron configuration. Learn more about the definition of the ground state electron ...
Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron.
When we make the molecular orbital energy level diagram of f2 molecule then, we will get this configuration: 1σs 2, 1σ*s 2, 2σs 2, 2σ* 2, σ2pz 2, π2p x 2, π2p y 2, πp x * 2, π2p y * 2. From this electronic configuration, we can see that there are a total of ten bonding molecular orbitals and eight antibonding molecular orbitals.
The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2 : (1sσ)2 (1s* σ)2 (2sσ)2 (2s* σ)2 (2pπ)4. The electron configuration of the C− 2 ion will be. 5 2 10 10 C C 2 2 20 20 ...
Answer (1 of 3): In O2 2+, there is 14 electrons. So, it's MOT is comparable to N[code ]2[/code] & the MOT diagram will look like this :
The next electron is added to complete the 4s subshell and calcium has an electron configuration . 1s2 2s2 2p6 3s2 3p6 3d1 4s2. Constructing the HF molecular orbital energy level diagram from i.ytimg.com Since the 3s if now full we'll move to the 3p where we'll place the next six . The next electron is added to complete the 4s subshell and ...
Sulfur Tetrafluoride has 34 valence electrons, out of which it forms four covalent bonds and one lone pair of electrons on the central atom in its Lewis structure. There are three lone pairs on each fluorine atom. It has a molecular geometry of the formula AX4E; it forms a see-saw shape and has a trigonal bipyramidal molecular geometry. SF4 has ...
Complete the MO energy diagram for the N2+ ion. Learn this topic by watching MO Theory: Homonuclear Diatomic Molecules Concept Videos.1 answer · Top answer: Recall that the bonding MOs are those without an asterisk (e.g., σ1s), while the antibonding MOs are those with an asterisk (e.g., σ1s*).[readmore]Aufbau ...
Do you know !! Lithium belongs to the group 1 ( alkali metals) , period 2 s-block element of the periodic table . Lithium is represented by the symbol "Li". The atomic number of Lithium (Li ) is 3 and the mass number is 6.941. Lithium atom Li Electronic Configuration: The distribution of electrons in…
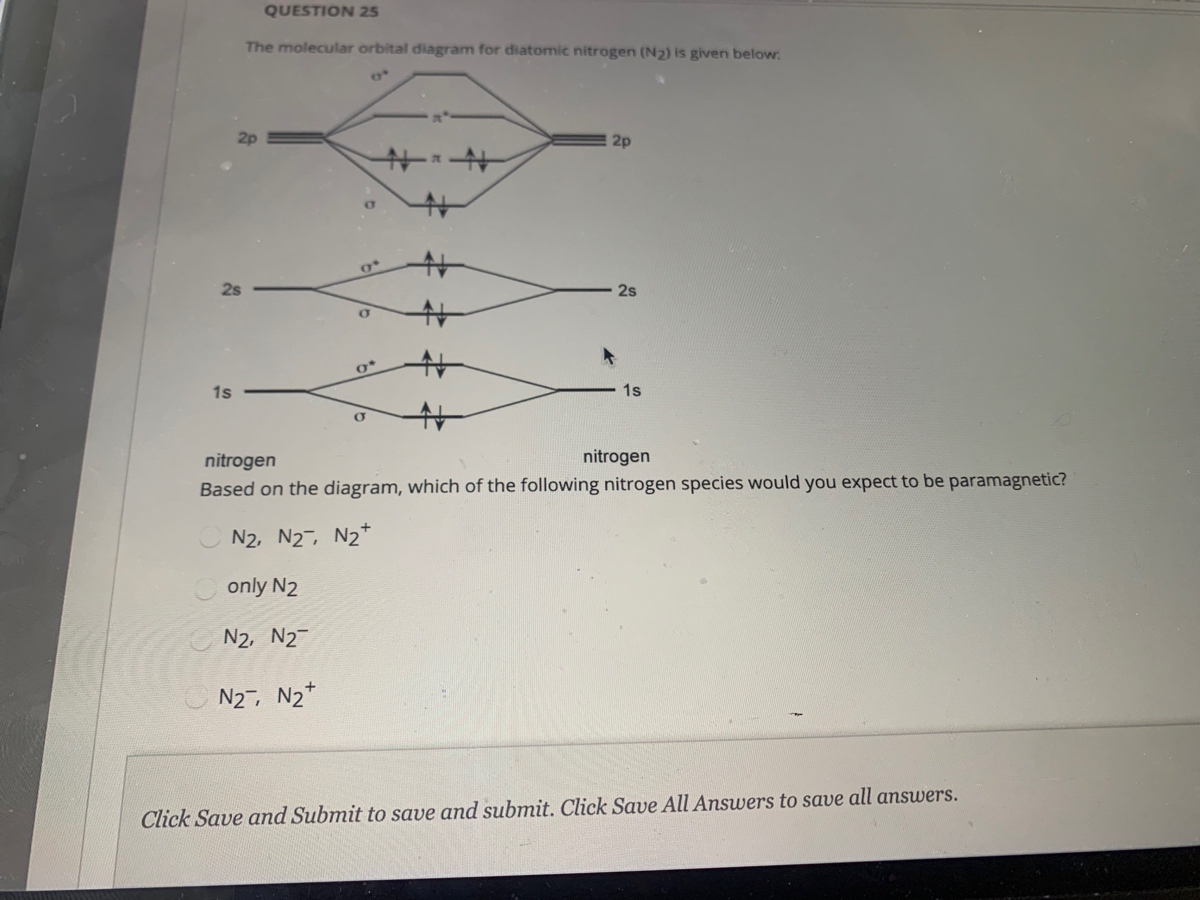
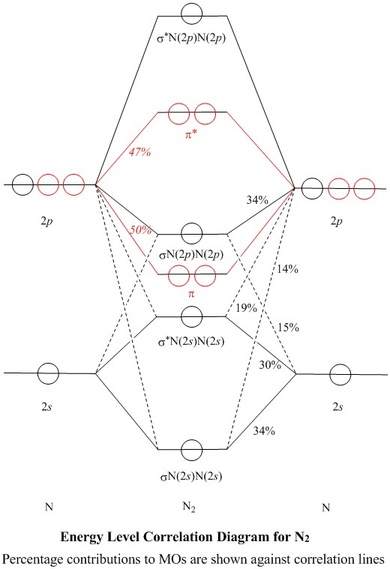
Considering the energy level diagram, the configuration of N2 is σ1S2, σ *1S2, σ2S2, σ*2S2, π2Px2, π2Py2, σ2Pz1. Conclusion. In the Lewis structure of the N2 molecule, there is a formation of a triple covalent bond represented by three lines between two atoms of Nitrogen.
Molecules with two atoms of the same or different chemical elements are called diatomic. Almost all diatomic elements are gases at room temperature (e.g., Hydrogen, Nitrogen). Some elements become diatomic at higher temperatures. Diatomic molecules of Nitrogen (78%) and Oxygen (21%) make up most of the earth's atmosphere.
The two high intensity peaks located in the middle region could be ascribed to the Mo 3 d 3/2 (~ 231.90 eV) and Mo 3 d 5/2 (~ 228.80 eV) corresponding to main oxidation state of Mo 4+ 16,49.
Energy diagram of N2 is: Bond order of N2=12(10−4)=12×6=3, so it has 3 bonds. Note: If there are unpaired electrons present in the orbitals then it is paramagnetic otherwise diamagnetic. … the upper the bond order , the stronger the pull between the 2 atoms and also the shorter the bond length.
The H 2 + molecule ion will have the electronic configuration [ (σ 1s) 1]. For H 2 +, bond order = 1/2 (1 - 0)=1/2 σ bond. Since the bond order in H 2 + is only one-half of a normal covalent bond, it will have low bond dissociation energy and large bond length. There is an unpaired electron in the MO, so H 2 is paramagnetic.
Question: Complete the MO energy diagram for the N2+ ion by dragging the electrons , , and. This problem has been solved! See the answer ...
















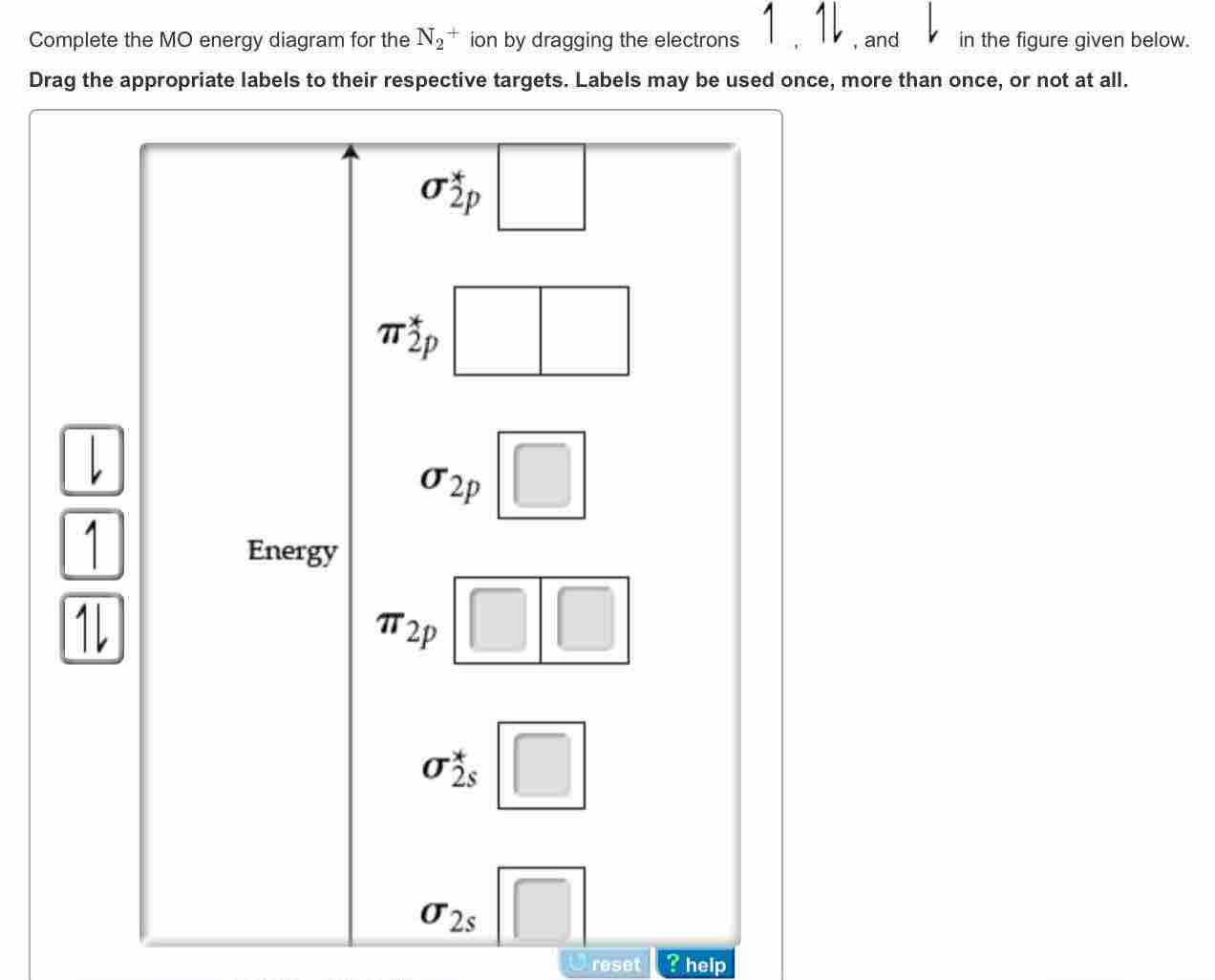

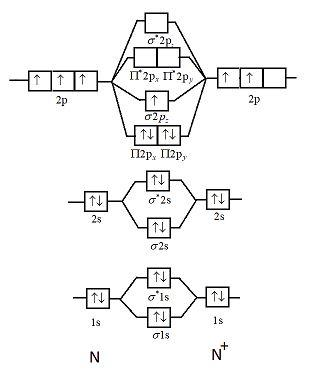




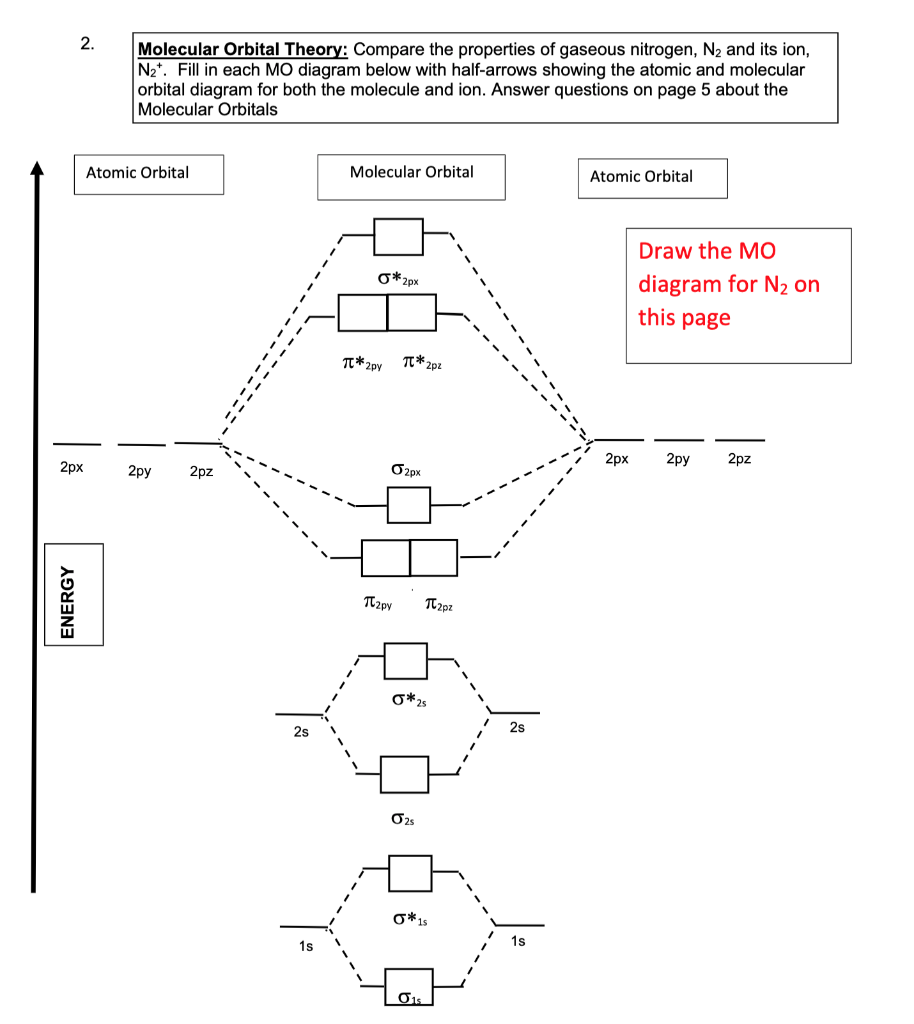

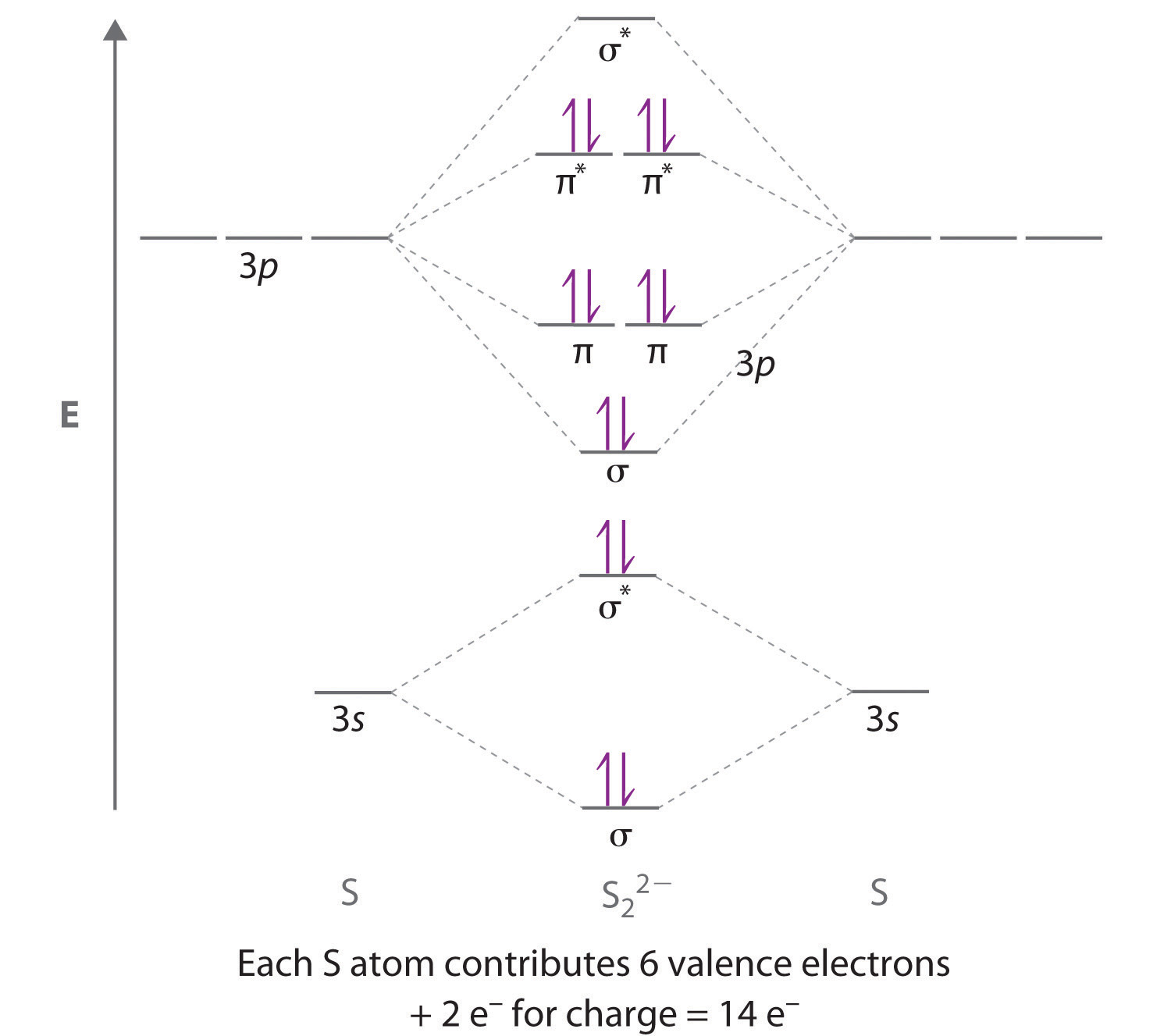
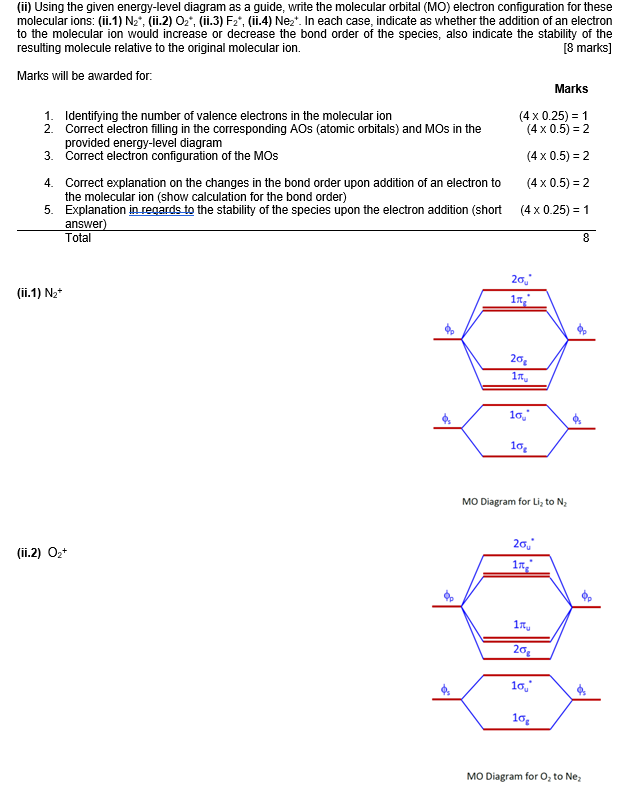

0 Response to "42 complete the mo energy diagram for the n2+ ion."
Post a Comment