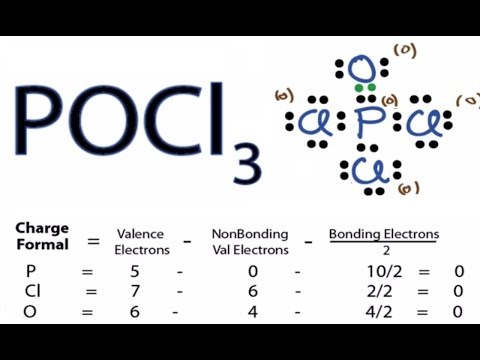
40 lewis dot diagram for pocl3
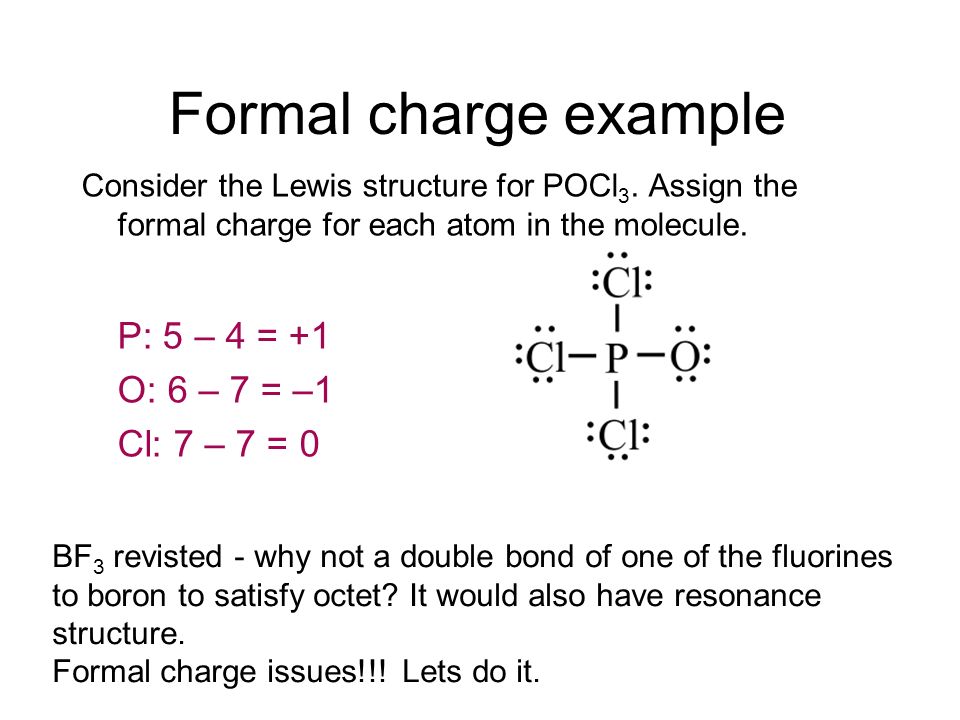
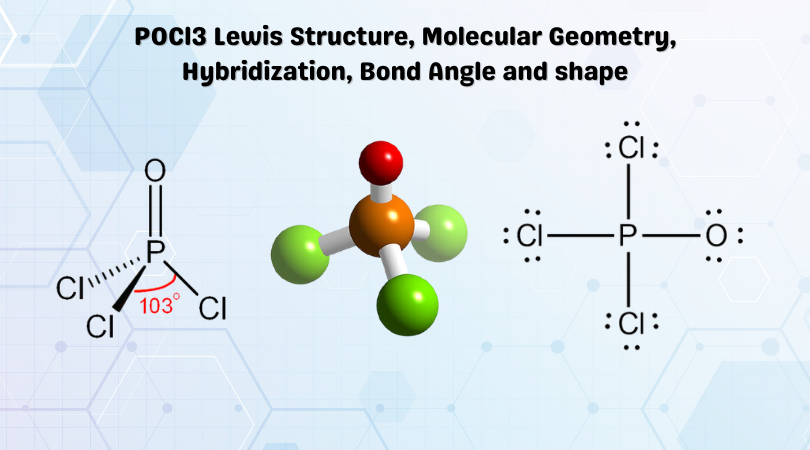
Re: Lewis structure for POCl3. P is the central atom because it is the least electronegative in comparison to O and Cl, and it also has the ability to be an expanded octet, which makes the drawing of the lewis structure accurate.
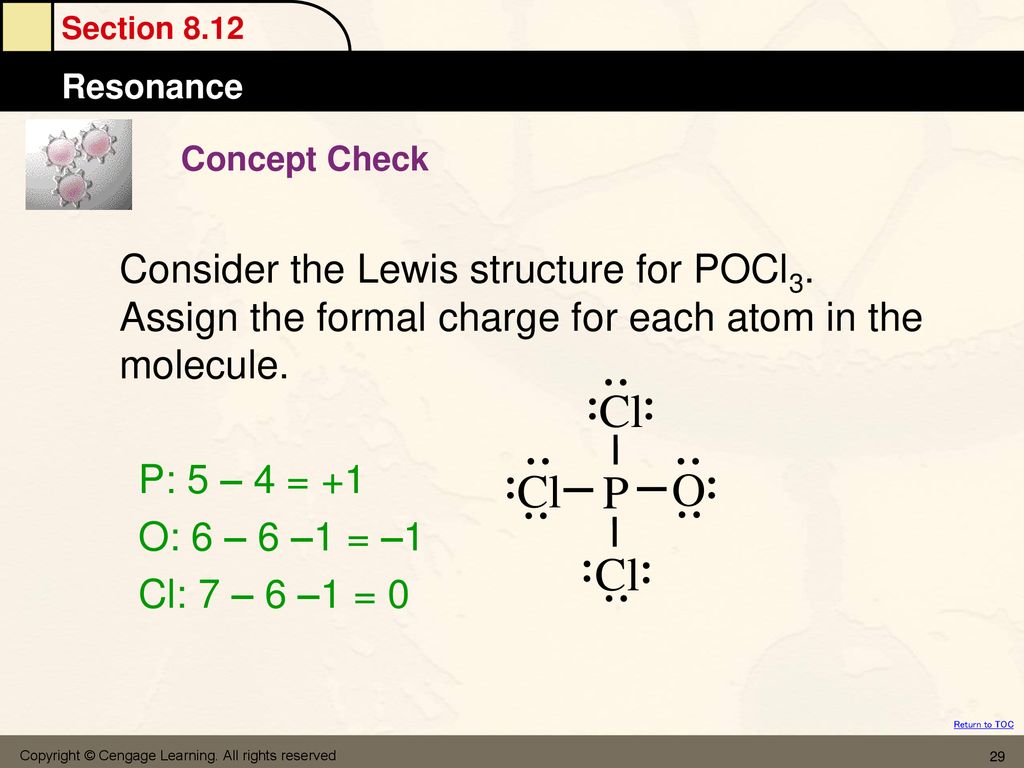
Re: Lewis Structure of POCL3? You would have to leave the P-O bond as a double bond with two lone pair electrons because when you calculate the formal charge, it would be zero at every atom. If you left it as a single bond with three lone pairs, Oxygen would have a FC of -1 and P would have a formal charge of 1.
A step-by-step explanation of how to draw the POCl3 Lewis Dot Structure (Phosphoryl chloride).For the POCl3 structure use the periodic table to find the tota...

Lewis dot diagram for pocl3
33 lewis dot diagram for pcl3 these pictures of this page are about:pocl3 lewis dot structure. The result is a lewis structure in which each atom has an octet of valence electrons. A simple procedure for writing lewis dot structures was given in a previous post entitled lewis structures and the octet rule.
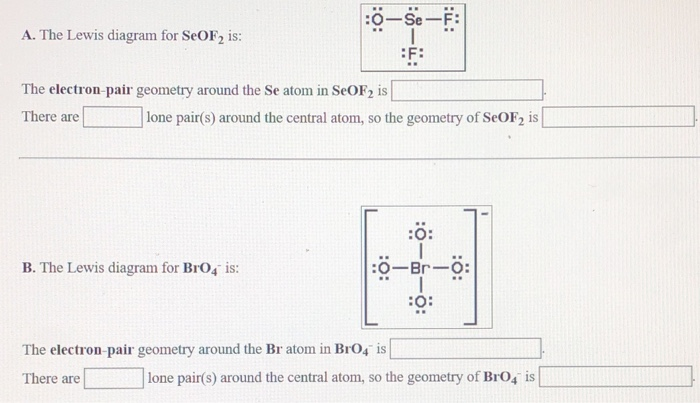
The Lewis diagram for POCl3 is: :Ci: The electron-pair geometry around the P atom in POCl is There are lone pair(s) around the central atom, so the geometry of POCl3 is :CI: B. The Lewis diagram for AlCl, is: AI Cl Cl The electron-pair geometry around the Al atom in AICl3 is There are lone pair(s) around the central atom, so the geometry of AICI3
POCl3 Lewis Structure. The Lewis structure of any molecule helps understand the arrangement of atoms in the molecule, its bond formation, and the valence electrons participating in forming bonds. The valence electrons that take part in forming bonds are called bonding pairs of electrons, whereas those that do not form bonds are called lone ...
Lewis dot diagram for pocl3.
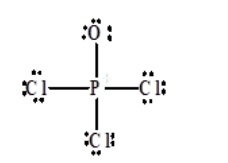
Phosphoryl chloride, POCl 3, has the skeleton structure: . Write: A. The Lewis structure for POCl 3 following the Octet rule. Calculate the formal charges in this structure. B. A Lewis structure in which all the formal charges are zero.
how to draw Lewis dot structures| POCl3| lewis resonance structures| drawi for, the periodic of elements, pocl3 lewis structure, draw the lewis structure of pocl3, dot.cross structure of pocl3, Chemistry Net This chemistry blog is aimed mainly at senior high school students or first year university students. ...
lewis dot structure of POCL3 2 See answers risheshshukla12 risheshshukla12 Answer: Mark Brainliest and Follow me . prathmeshshreypch9ul prathmeshshreypch9ul Answer: Step 1: Connect the atoms with single bonds. The less electronegative is the phosphorous atom. Hence, the P atom is going to be the central atom.
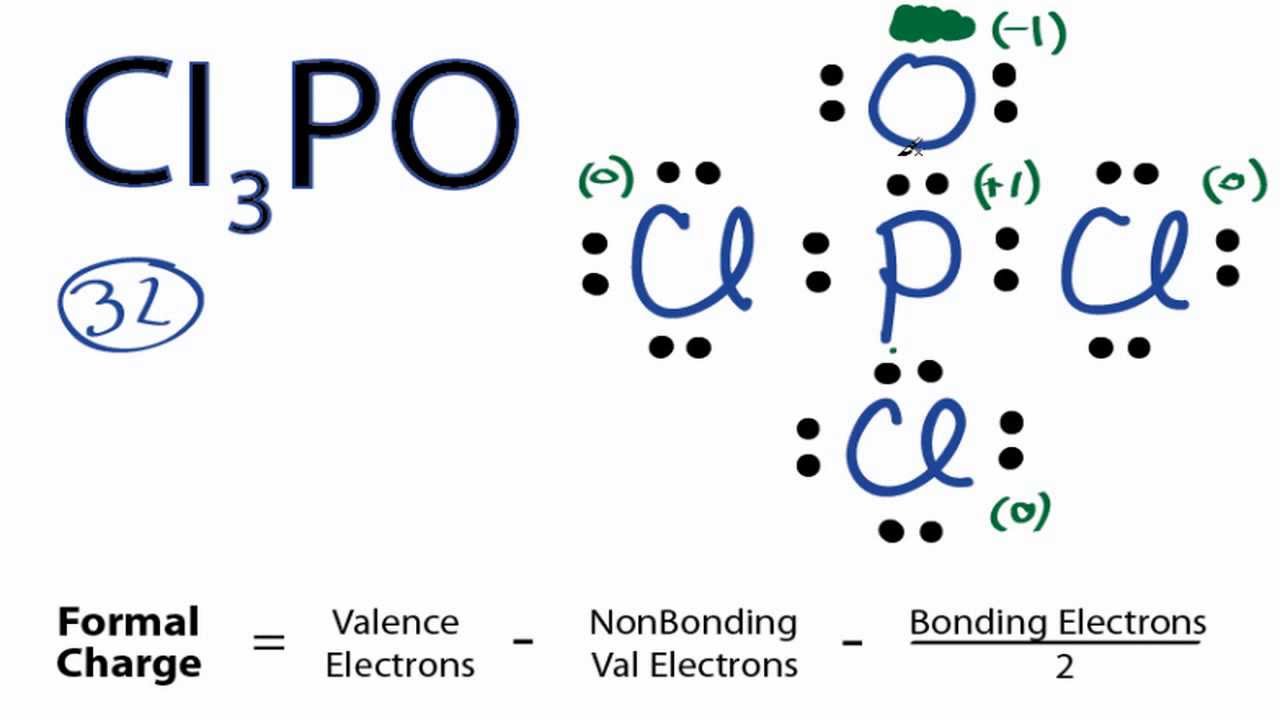
In the POCl 3 Lewis structure Phosphorus (P) is the least electronegative so it goes in the center. In the Lewis structure for POCl 3 there are a total of 32 valence electrons. If you check the formal charges for POCl 3 you'll find that you need a double bond between the Phosphorous and Oxygen atom in order to have the formal charges equal zero.






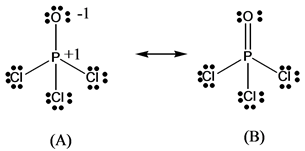





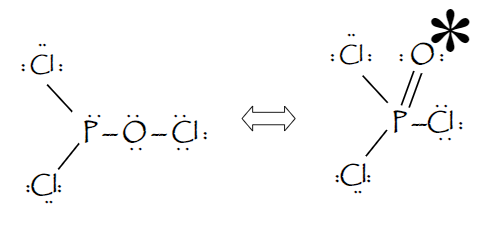














0 Response to "40 lewis dot diagram for pocl3"
Post a Comment