39 diagram of exothermic reaction
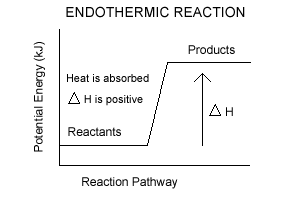
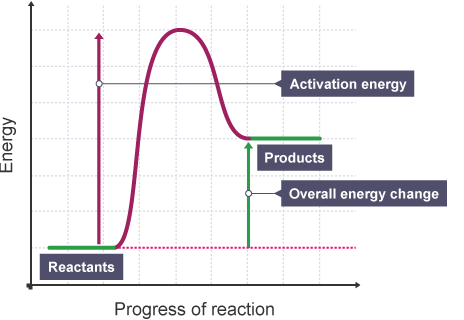
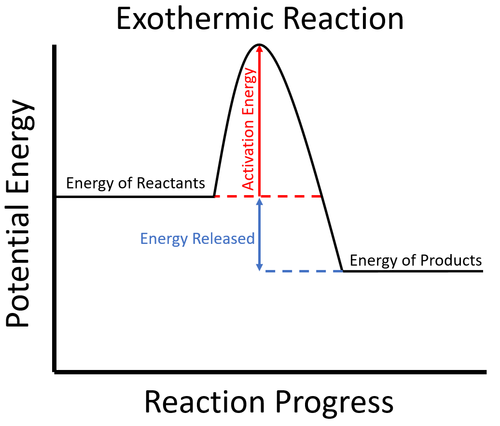
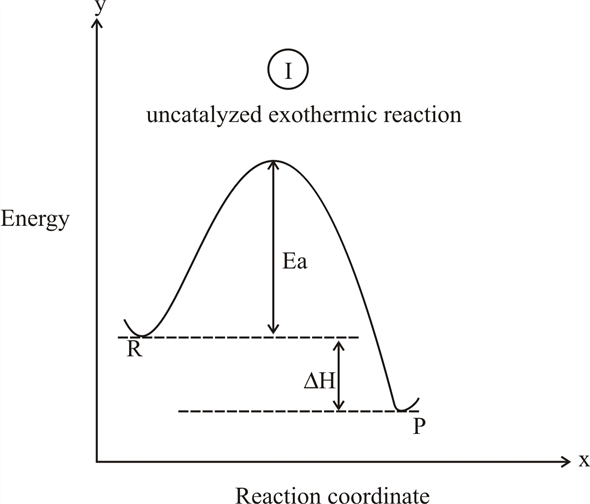
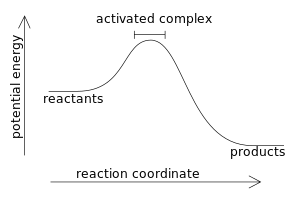
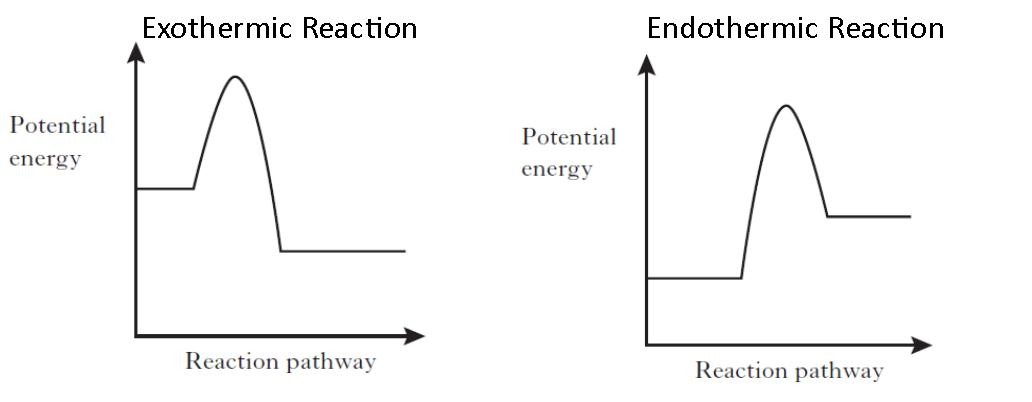
An exothermic reaction liberates heat, temperature of the reaction mixture increases. An endothermic reaction absorbs heat, temperature of the reaction mixture decreases. The units of enthalpy of reaction, or heat of reaction, are kJ mol -1 for a specified reactant or product. Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react.
A heat absorption reaction is endothermic. Its enthalpy will be positive, and its surroundings will cool down. This reaction (negative enthalpy, heat release) is exothermic. When the reaction happens, due to the gain in heat the device emits, the atmosphere may rise in temperature.

Diagram of exothermic reaction
Jul 9, 2021 — Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ... In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below. 02/12/2016 · Exothermic Reaction Endothermic Reaction 49. Factors Affecting Rate Of Reaction - Explanation By Collision Theory Total Surface Area of Reactants • When the size of the solid substance that reacts is smaller, its total surface area exposed becomes larger. • This causes the collisions frequency between the reactants increases.
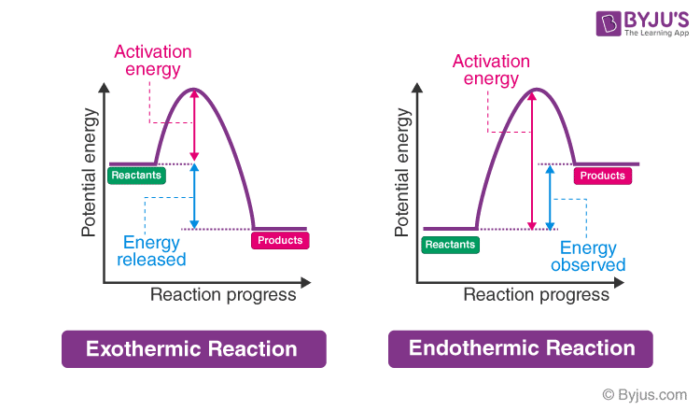
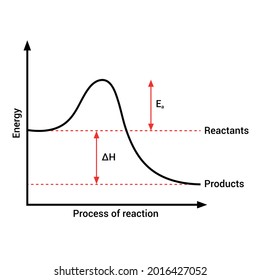
Diagram of exothermic reaction. For an exothermic reaction, the enthalpy change is always negative. An energy level diagram for an endothermic reaction. In an endothermic reaction, the products are at a higher energy than the ... In an exothermic reaction, energy is released because the total energy of the products is less than the total energy of the reactants. For this reason, the change in enthalpy, [latex]\Delta H[/latex], for an exothermic reaction will always be negative. The energy level decreases in an exothermic reaction. This is because energy is given out to the surroundings. Graph of energy against progress of reaction. In an addition reaction the number of σ-bonds in the substrate molecule increases, usually at the expense of one or more π-bonds. The reverse is true of elimination reactions, i.e.the number of σ-bonds in the substrate decreases, and new π-bonds are often formed.Substitution reactions, as the name implies, are characterized by replacement of an atom or group (Y) by another atom …
Exothermic reaction In an exothermic reaction, the total energy of the products is less than the total energy of the reactants. Therefore, the change in enthalpy is negative, and heat is released to the surroundings. Endothermic Reactions. Endothermic reactions are reactions that require external energy, usually in the form of heat, for the ... The energy level diagram helps to provide further understanding of the propagation process. The 1 st step in propagation is endothermic, while the energy absorbed can be offset by the 2 nd exothermic step. Therefore the overall propagation is exothermic process and the products are in lower energy level the than reactants. ENERGY IS A REACTANT, SO THE REACTION IS ENDOTHERMIC AND ΔH IS POSITIVE! Page 5. EQUATIONS &. ENERGY DIAGRAMS. • WE CAN USE AN ENERGY DIAGRAM ...11 pages History. The process was invented by Carl Friedrich Claus, a German chemist working in England.A British patent was issued to him in 1883. The process was later significantly modified by IG Farben.. Claus was born in Kassel in the German State of Hesse in 1827, and studied chemistry in Marburg before he emigrated to England in 1852. He died in London in 1900.
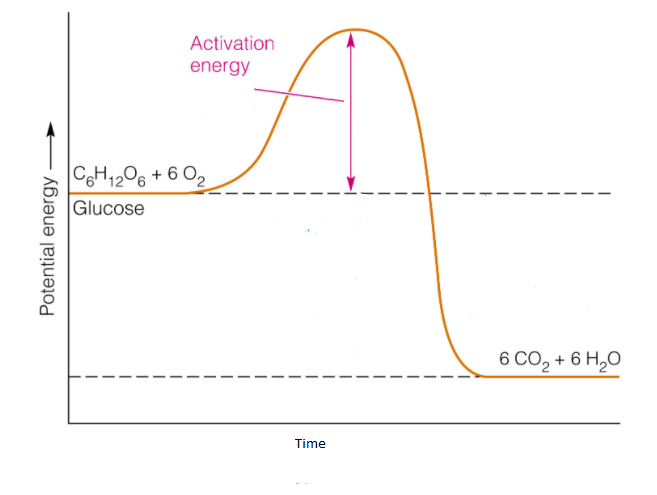
Apr 09, 2018 · 1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process. Base your answers on the information and diagram below, which represent the changes in potential energy that occur during the given reaction. Given the reaction: A + B --> C. a) Does the diagram illustrate an exothermic or an endothermic reaction? State one reason, in terms of energy, to support your answer. Endothermic Reaction Diagram | energy diagram for a catalyzed endothermic reaction ... Chemistry 30 Chemical Kinetics - Potential Energy Diagrams Revisited. A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation.
02/12/2016 · Exothermic Reaction Endothermic Reaction 49. Factors Affecting Rate Of Reaction - Explanation By Collision Theory Total Surface Area of Reactants • When the size of the solid substance that reacts is smaller, its total surface area exposed becomes larger. • This causes the collisions frequency between the reactants increases.
In the case of an endothermic reaction, the reactants are at a lower energy level compared to the products—as shown in the energy diagram below.
Jul 9, 2021 — Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ...
























0 Response to "39 diagram of exothermic reaction"
Post a Comment