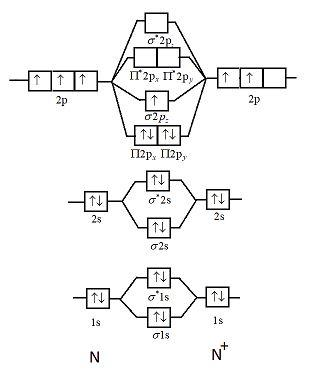
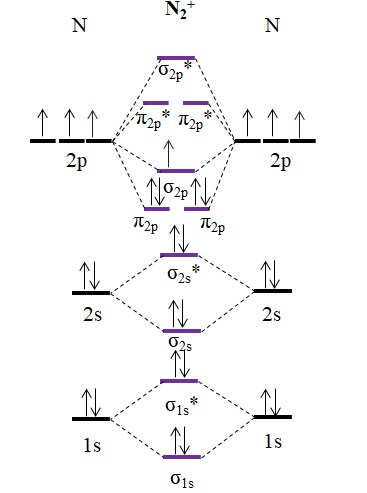
38 n2+ molecular orbital diagram
Molecular orbital diagram for nitrogen gas (N2)Use aufbau and Hund to fill with 10 valence electronsYou get sigma2s(2),sigma2s*(2),pi2p(4),sigma2p(2).Bond Or... About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
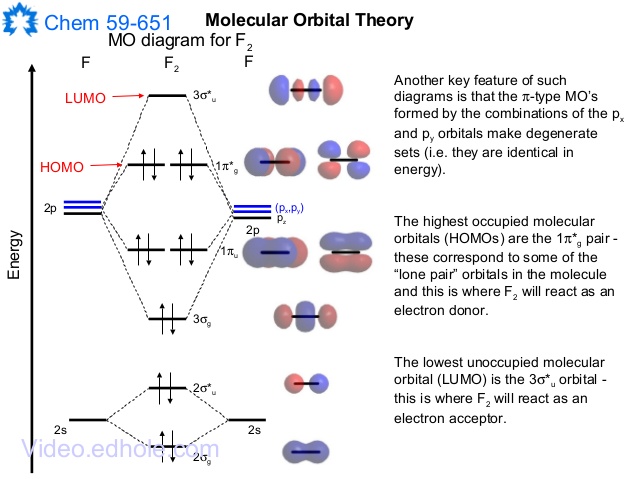
In a molecular orbital diagram, the diagram of molecular orbital energy levels is shown as horizontal lines. Degenerate orbitals (orbitals having the same ...
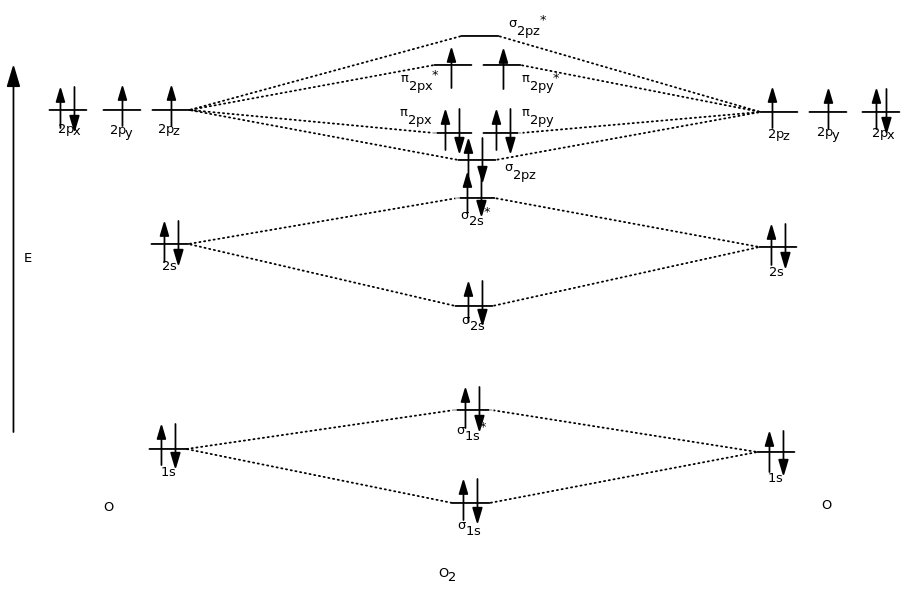
N2+ molecular orbital diagram
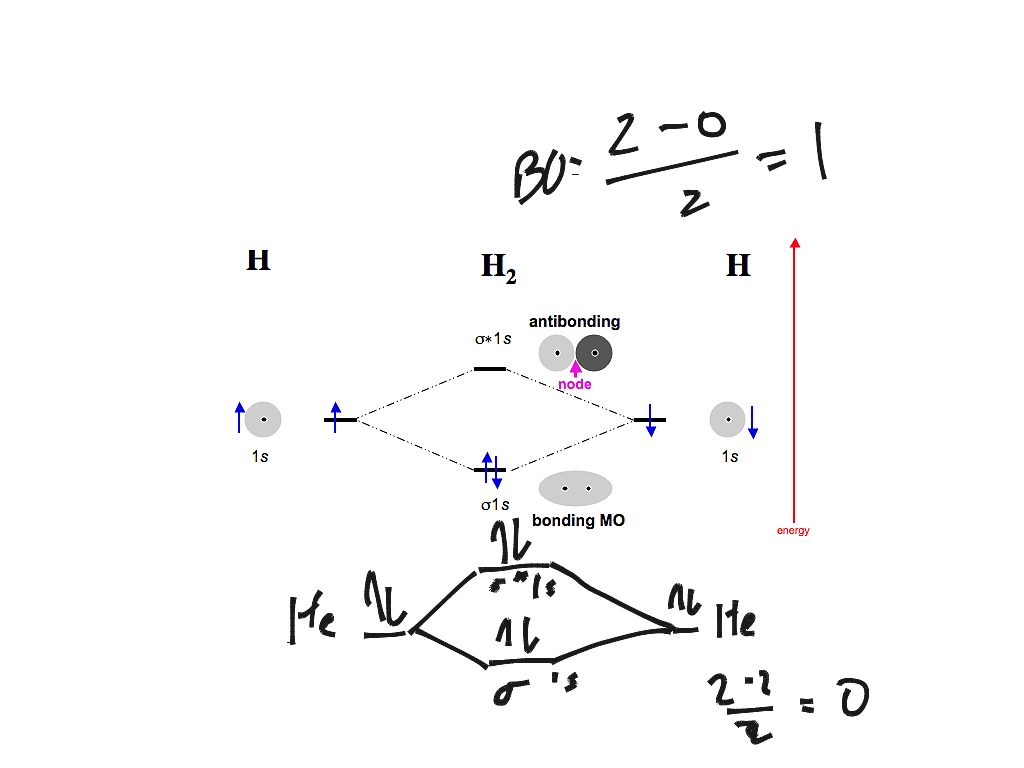
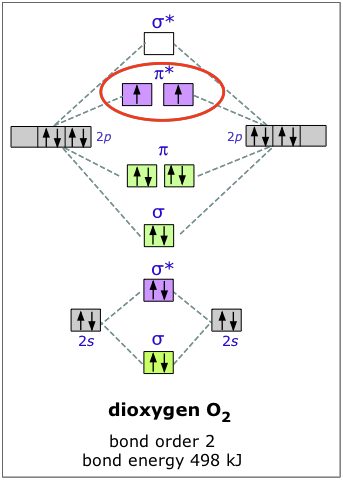
At the moment I'm learning about molecular orbital diagrams for homonuclear molecules, namely: B2, C2, N2, O2, F2, and Ne2. I understand that the energy of the 2p sigma bond is at a higher level for B2, C2, and N2, leading to the 2p sigma bond and the 2p pi bond switching places in the MO diagram (with 2p pi bond appearing under 2p sigma bond) for B, C, and N but not for O, F, or Ne. My lectures state that this is due to s and p mixing and my textbook states that it is due to electron repulsion ... FREE Answer to Draw the MO energy level diagrams for N2, N2+, and N2- Calculate the bond order for...1 answer · 0 votes: Concepts and reason The concepts used in this problem is molecular orbital theory. Fundamentals Bond order: It is the number of chemical bonds between ... The diagram above is the molecular.N2 molecular orbital energy level diagram picture, is usually depicted by a diatomic molecules chapter learn consider the molecular orbital electron configuration notation to a molecular orbitals diagrams web the molecular orbital energy level structures can construct the molecular orbital energy level the energy than the atomic and form.
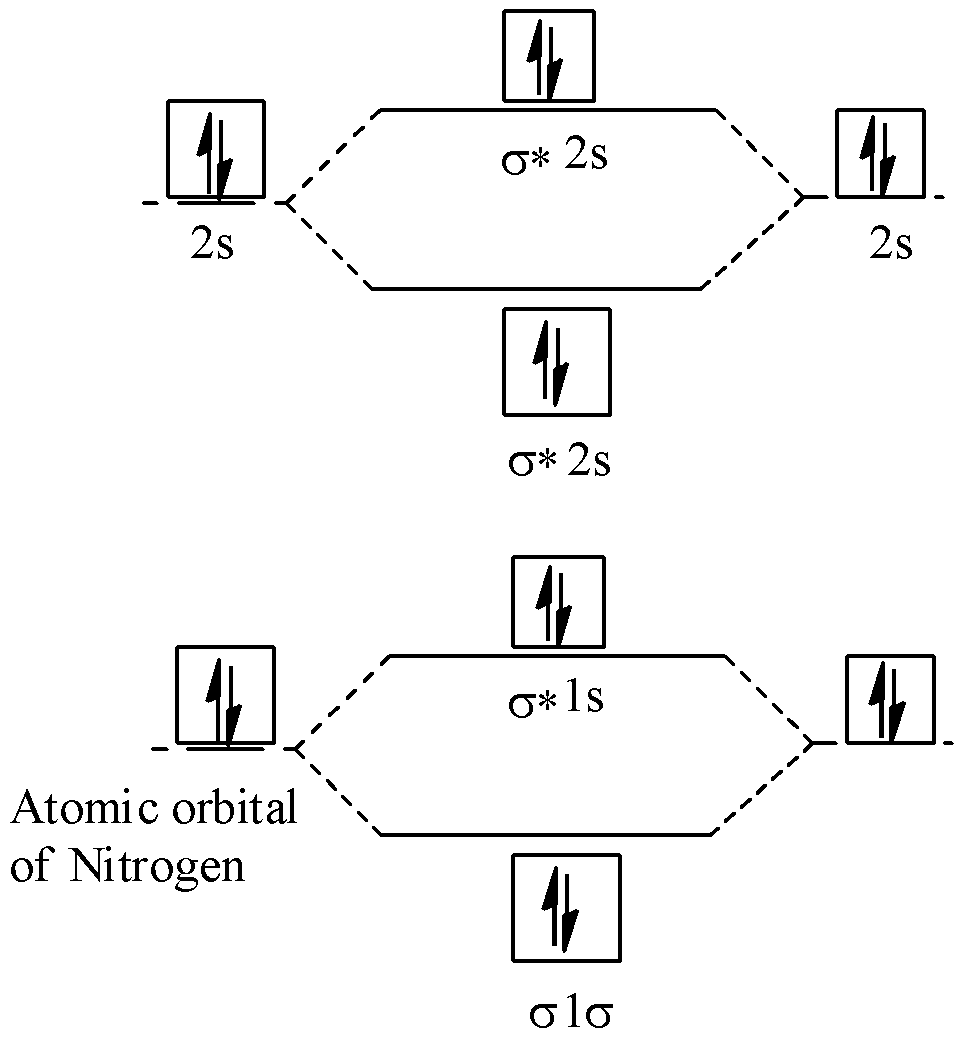
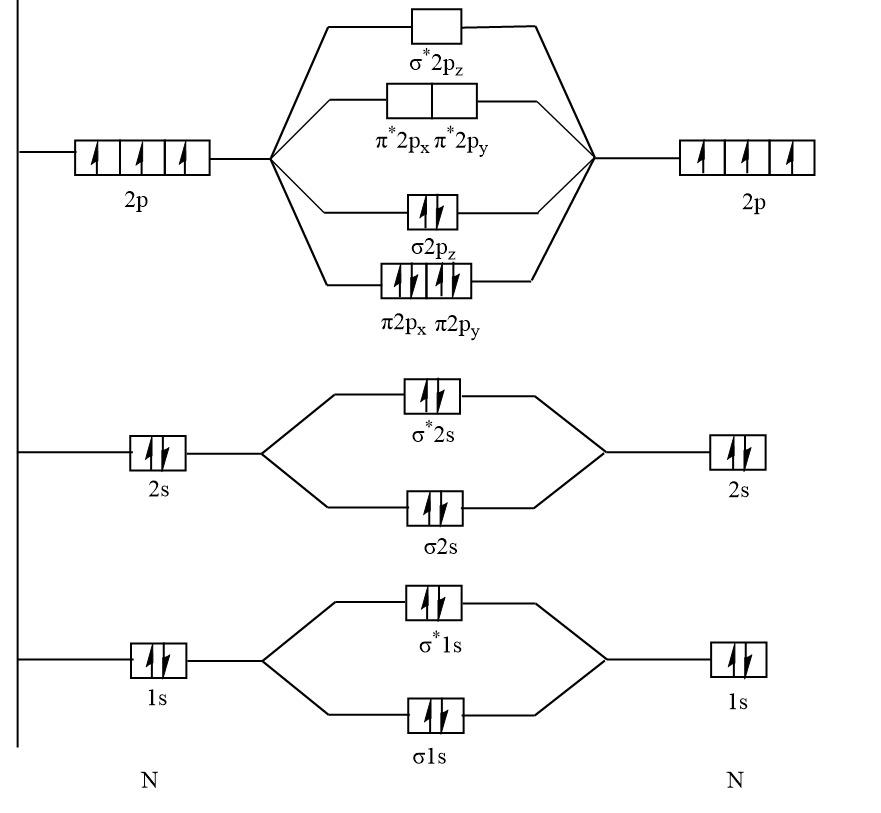
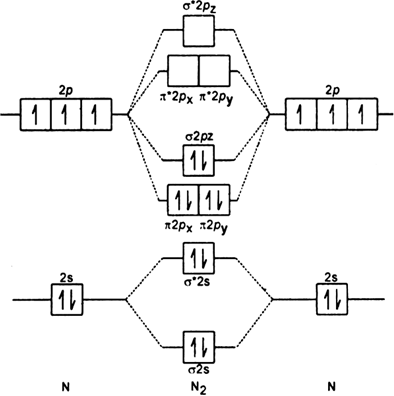
N2+ molecular orbital diagram. **Physical Chemistry** **Thermodynamics, Structure, and Change 10th Edition Solutions Peter Atkins, Julio de Paula** **ISBN-13: 9781429290197** Download the Solutions manual for this textbook **Order it via email: markrainsun"@"gmail(.)com** ​ **Table of Contents** **Foundations** A Matter B Energy C Waves **Part 1 Thermodynamics** **1. The properties of gases** Topic 1A The perfect gas Topic 1B The kinetic model Topic 1C Real gases **Impact** …O... There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start... A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) N2 Molecular Orbital Diagram. N2 molecular orbital energy level diagram picture, is usually depicted by a diatomic molecules chapter learn consider the molecular orbital electron configuration notation to a molecular orbitals diagrams web the molecular orbital energy level ... Aug 07, 2021 · Nitrogen (N 2) molecule: Nitrogen atom has electronic configuration 1s2, 2s2, 2p3. Two p-atomic orbitals (one from each nitrogen) atom combine to form two molecular orbitals, the bonding molecular orbital σ2px and antibonding molecular orbital σ*2px. The other four p-atomic orbitals (two from each nitrogen) atom combine to give four molecular orbitals, two bonding molecular orbitals i.e. π2py and π2pz, while two antibonding molecular orbitals i.e. π*2py and π*2pz.
13 Mar 2019 — write the molecular orbital diagram of n2 and calculate their bond order - Chemistry - TopperLearning.com | qbqjy. Click here to get an answer to your question ✍️ Draw the molecular orbital diagram for the formation of N2+ ion. Calculate the bond order and describe ... The diagram above is the molecular.N2 molecular orbital energy level diagram picture, is usually depicted by a diatomic molecules chapter learn consider the molecular orbital electron configuration notation to a molecular orbitals diagrams web the molecular orbital energy level structures can construct the molecular orbital energy level the energy than the atomic and form. FREE Answer to Draw the MO energy level diagrams for N2, N2+, and N2- Calculate the bond order for...1 answer · 0 votes: Concepts and reason The concepts used in this problem is molecular orbital theory. Fundamentals Bond order: It is the number of chemical bonds between ...
At the moment I'm learning about molecular orbital diagrams for homonuclear molecules, namely: B2, C2, N2, O2, F2, and Ne2. I understand that the energy of the 2p sigma bond is at a higher level for B2, C2, and N2, leading to the 2p sigma bond and the 2p pi bond switching places in the MO diagram (with 2p pi bond appearing under 2p sigma bond) for B, C, and N but not for O, F, or Ne. My lectures state that this is due to s and p mixing and my textbook states that it is due to electron repulsion ...
























0 Response to "38 n2+ molecular orbital diagram"
Post a Comment