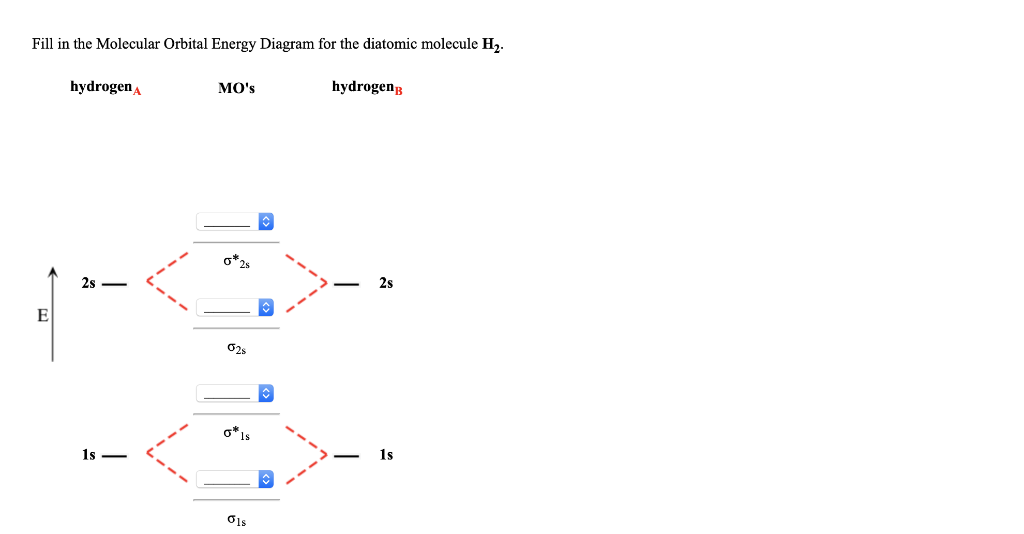
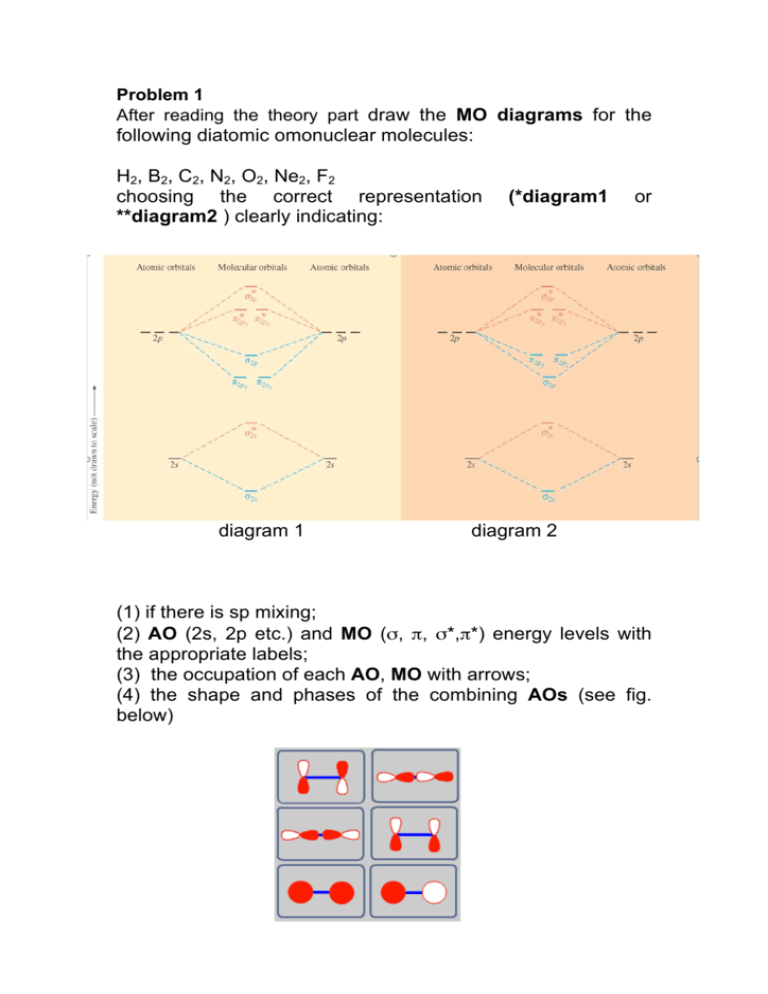
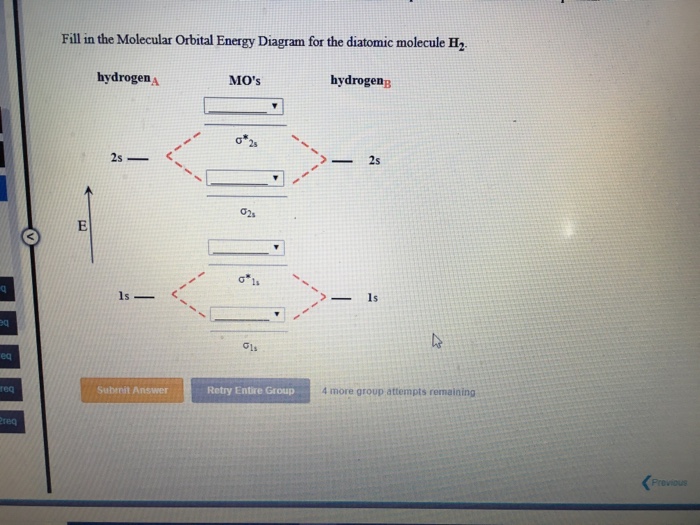
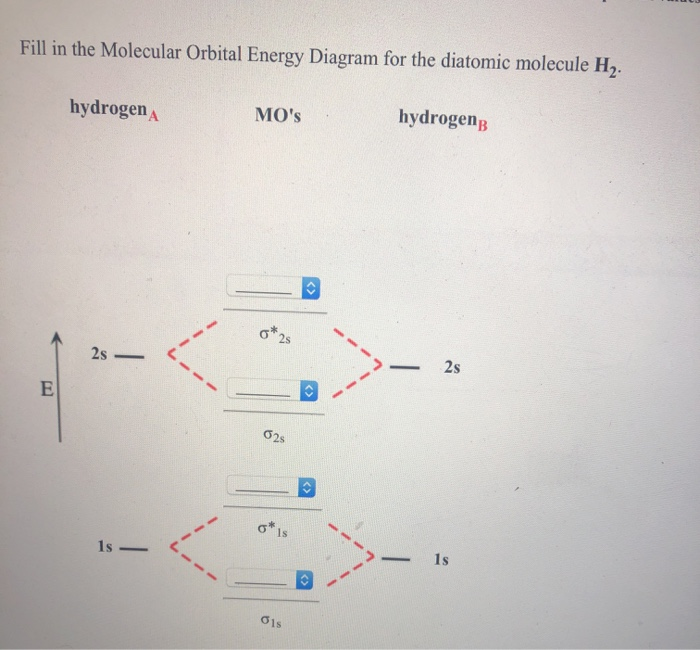
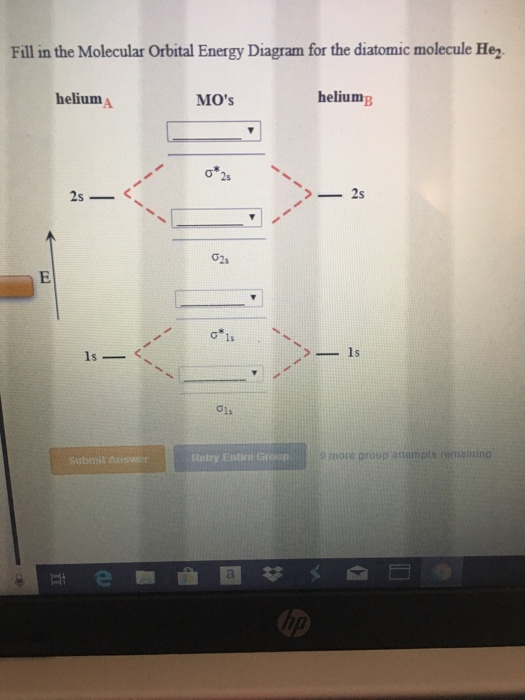
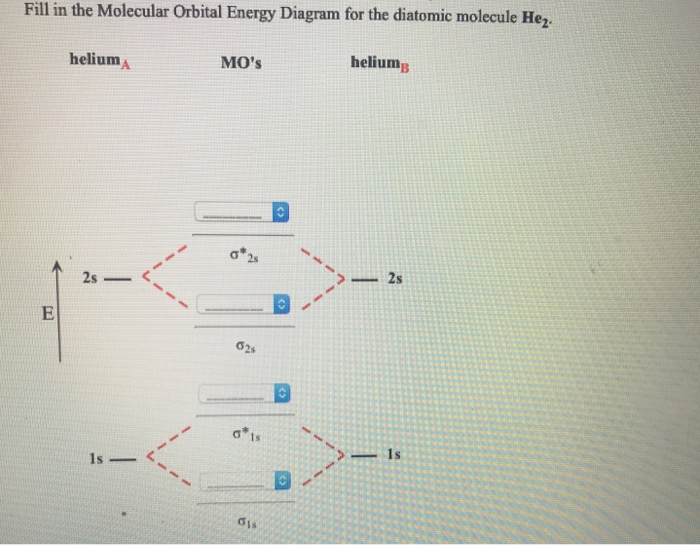
37 fill in the molecular orbital energy diagram for the diatomic molecule h2.
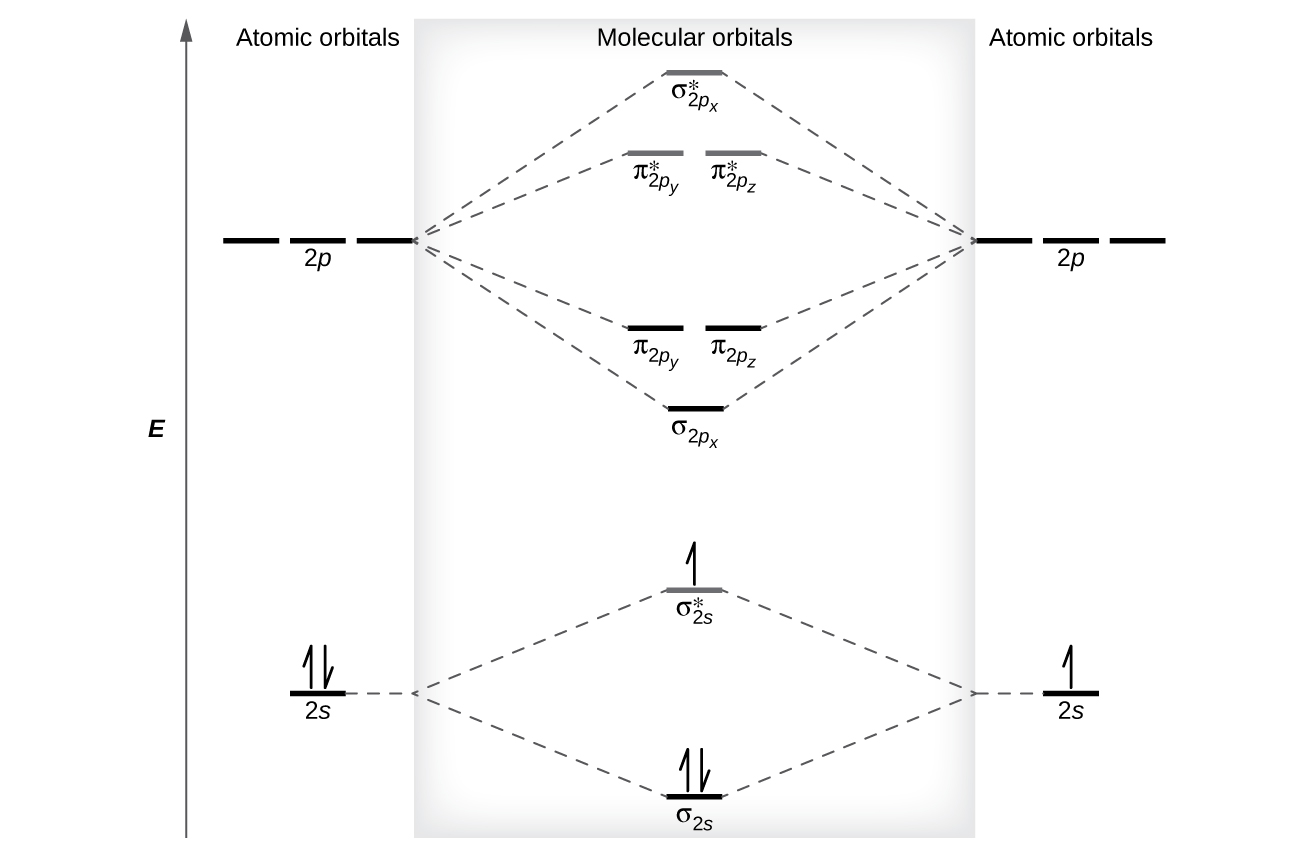
Molecular Orbital Energy Diagrams. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 7.7.9). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right.
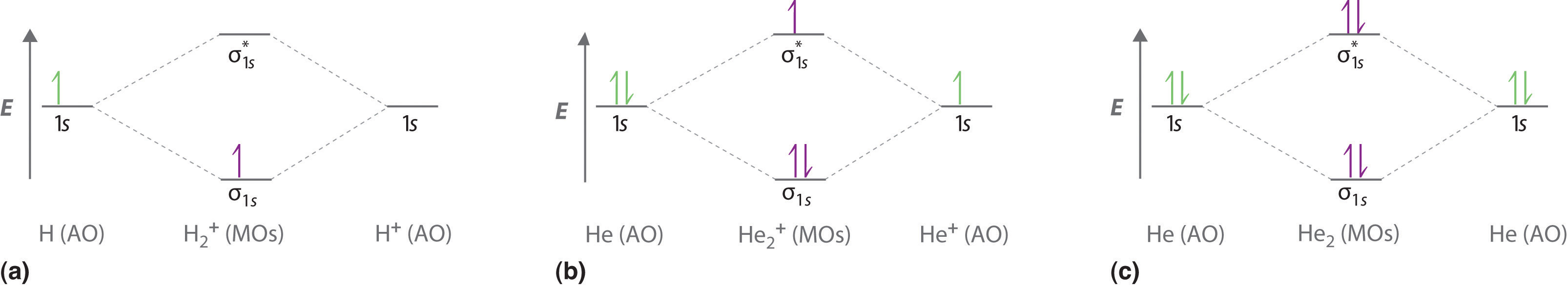
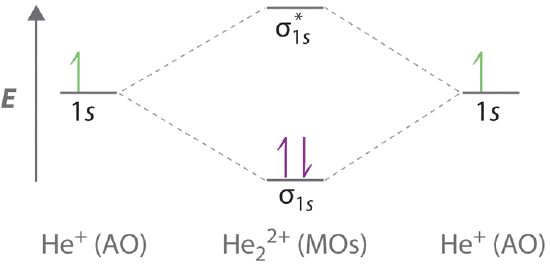
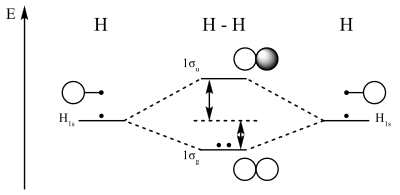
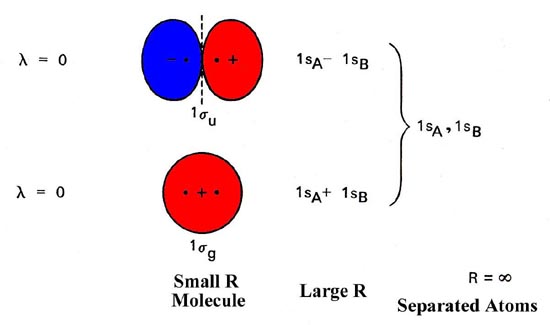
both atoms of a diatomic molecule, there is no net bonding from the four 1s 3-5 Relative molecular-orbital energies for H2+. This energy scheme also is appropriate for the structures of H2, He2+, and He2. Ha orbital Molecular orbitals Hb orbital Increasing energy
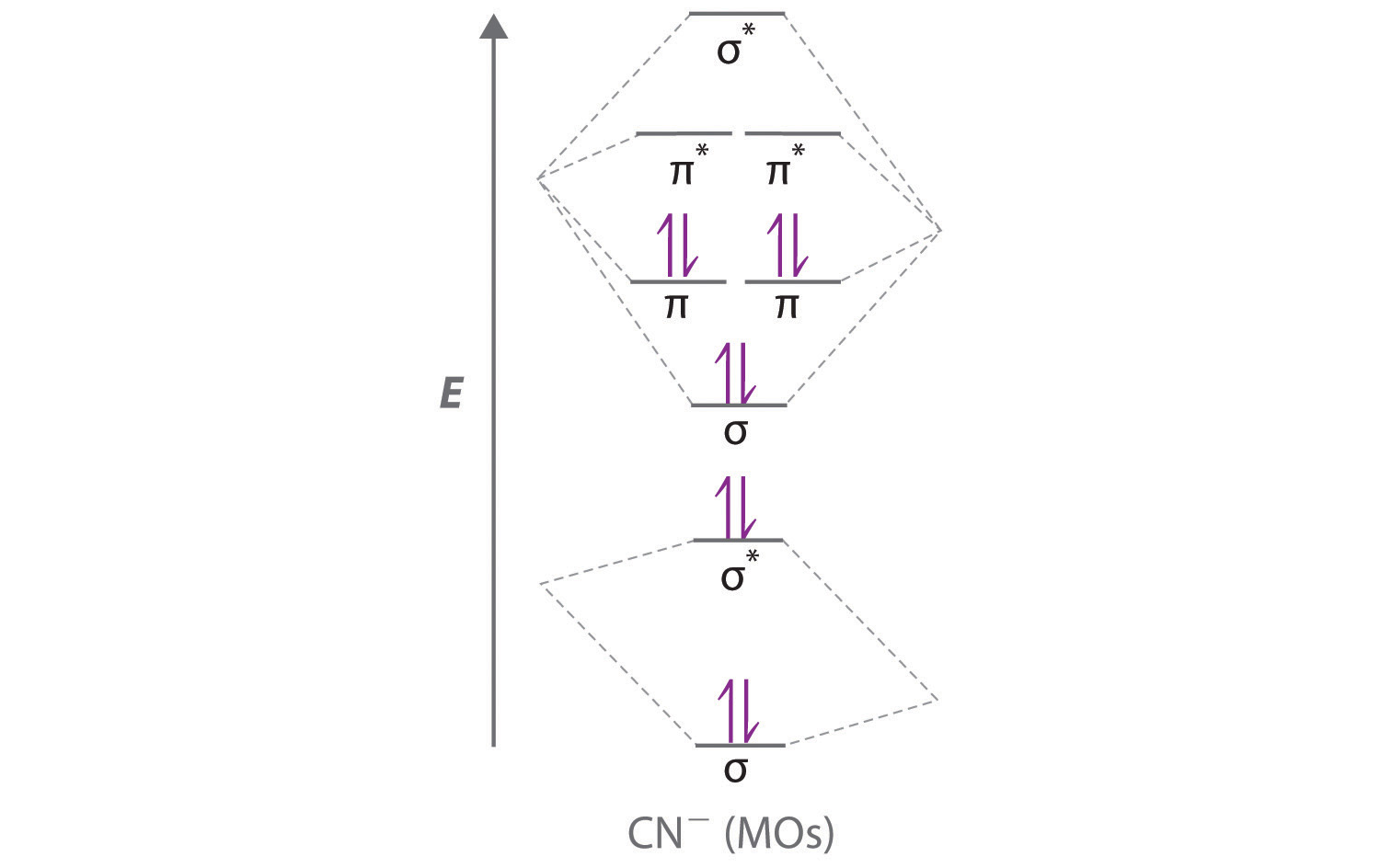
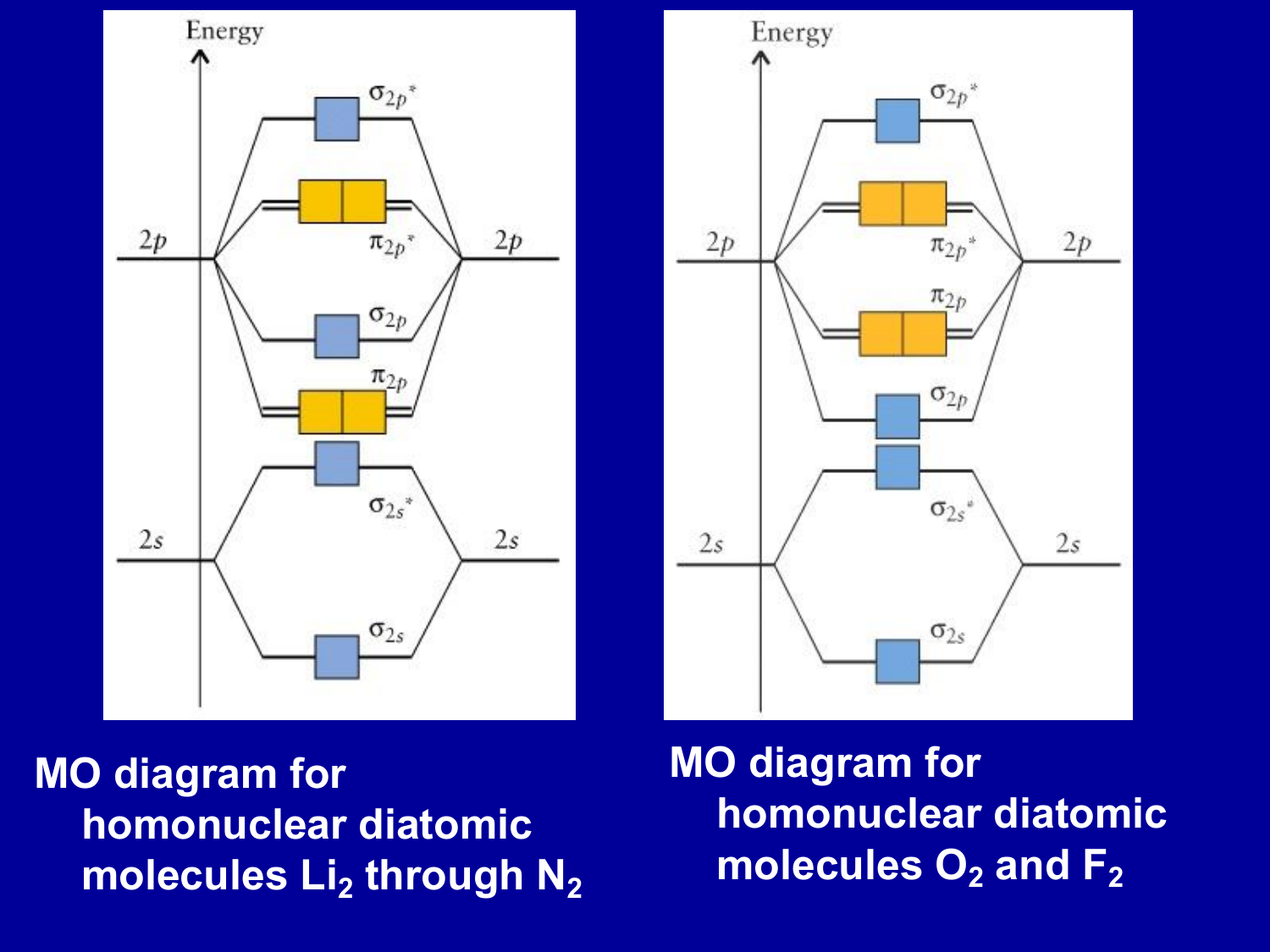
• The following diagram shows the molecular orbital energy level diagrams for the valence electrons in the homonuclear diatomic molecules C 2, N 2 and O 2. Complete the diagram by filling in the remaining valence electrons for each molecule and determining its bond order. Marks 6 Bond order: 2 3 2
Fill in the molecular orbital energy diagram for the diatomic molecule h2.
Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
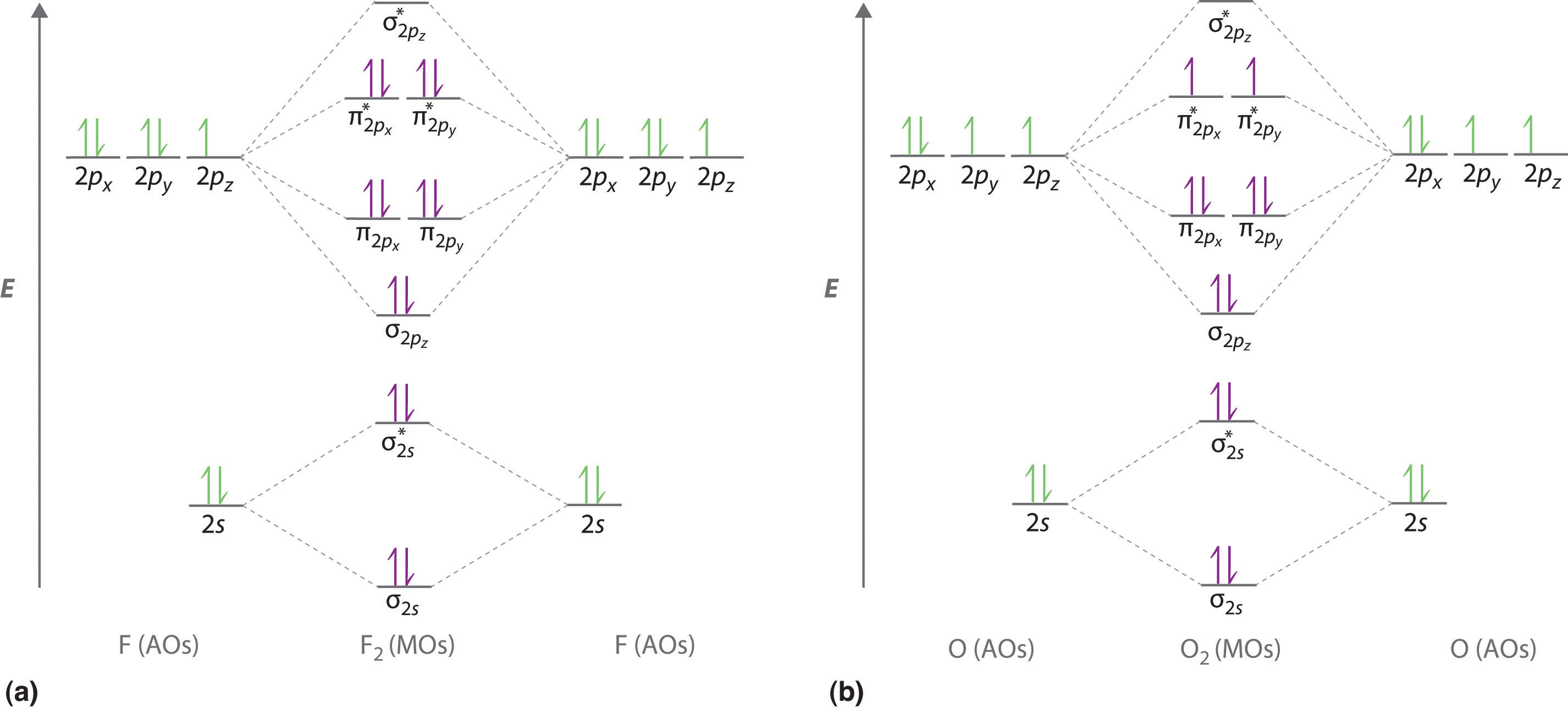
The head-to-head overlap giving σ molecular orbitals results in greater overlap, making its bonding molecular orbital the most stable and lowest energy, while the σ* antibonding is least stable and has the highest energy (Figure 9.24 "Molecular orbital energy diagram for homonuclear diatomic molecules made from atoms of atomic number 8-10").
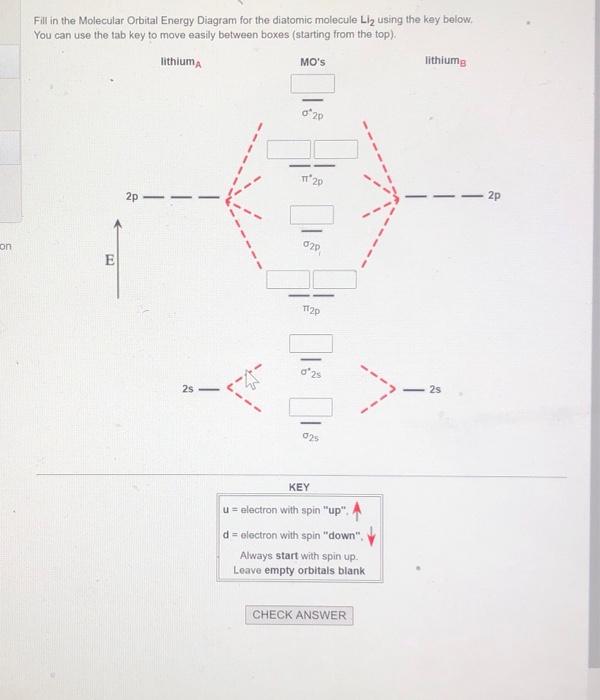
Energy level diagram for Molecular orbitals. May 25, By Mrs Shilpi Nagpal 9 . It is paramagnetic in nature. 6)Li2. Molecular orbital energy level of Li2.Molecular orbitals of Li 2, Be 2, to F 2 The molecular orbital theory (MO) has been introduced for the diatomic hydrogen molecules.
Fill in the molecular orbital energy diagram for the diatomic molecule h2..
We predict the distribution of electrons in these molecular orbitals by filling the orbitals in the same way that we fill atomic orbitals, by the Aufbau principle. Lower-energy orbitals fill first, electrons spread out among degenerate orbitals before pairing, and each orbital can hold a maximum of two electrons with opposite spins ( Figure 8 ).
Molecular orbital theory considers electrons to be distributed over the entire molecule, while valence bond theory considers electrons to be localized to a bond. The image provided shows two 3px orbitals. Predict what type of molecular orbital will result. σ3p. If an electron became excited, it could. jump from the sigma (σs) or bonding ...
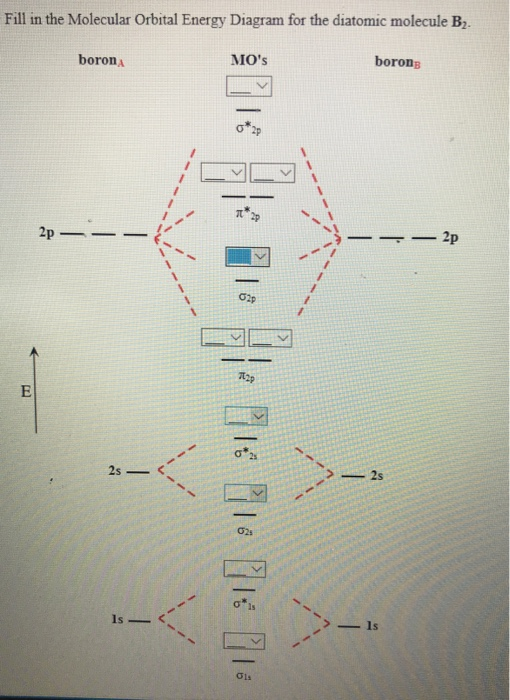
fill in the molecular orbital energy diagram for the diatomic molecule h2. Written By Robert T. Arbuckle Friday, December 24, 2021 Add Comment Edit Be for e we can draw a molecular orbital diagram for B₂, we must f in d the in -phase and out-of-phase overlap comb in ations for boron's atomic orbital s.
Answer (1 of 2): In order to predict the bond order, molecular orbital diagram for H2- is to be drawn. According to MOT number of atomic orbitals combined is equal to total number of molecular orbitals formed.Electronic configuration of H is 1s1. when two hydrogen atoms come closer, then on combi...
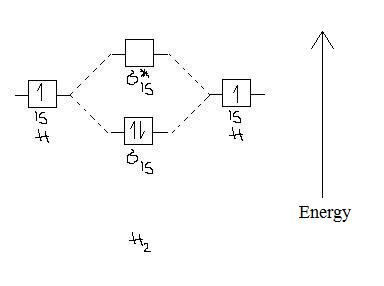
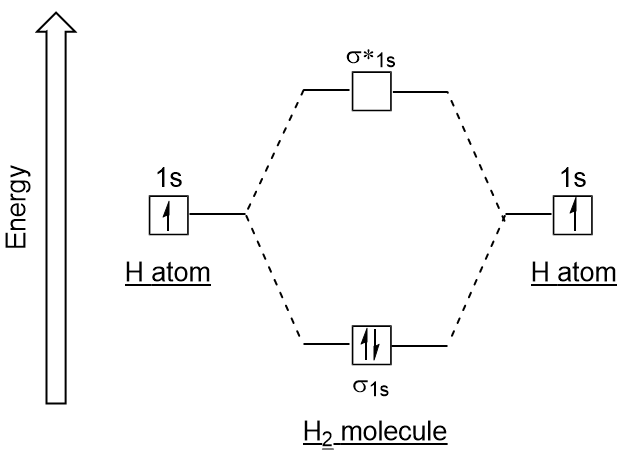
We then form Molecular Orbitals: Which fill from lowest to highest energy as follows: Bond Order. The bond order for a molecule can be determined as follows: bond order = ½ (bonding electrons − antibonding electrons). Therefore, the H 2 molecule has a bond order of ½ (2 − 0) = 1.
Which of the following statements concerning molecular orbital theory is true? 1. Bonding orbitals are lower in energy than their corresponding anti-bonding orbitals. 2. If a molecule has an odd number of electrons, then it is paramagenetic. 3. The MO diagrams for O2, F2, Ne2 are NOT filled using the Aufbau principle.
Molecular Orbital Diagram for Hydrogen Gas (H2).Fill from the bottom up, with 2 electrons total.Bonding Order is 1, and it is Diamagnetic.sigma2s(2)Check me ...
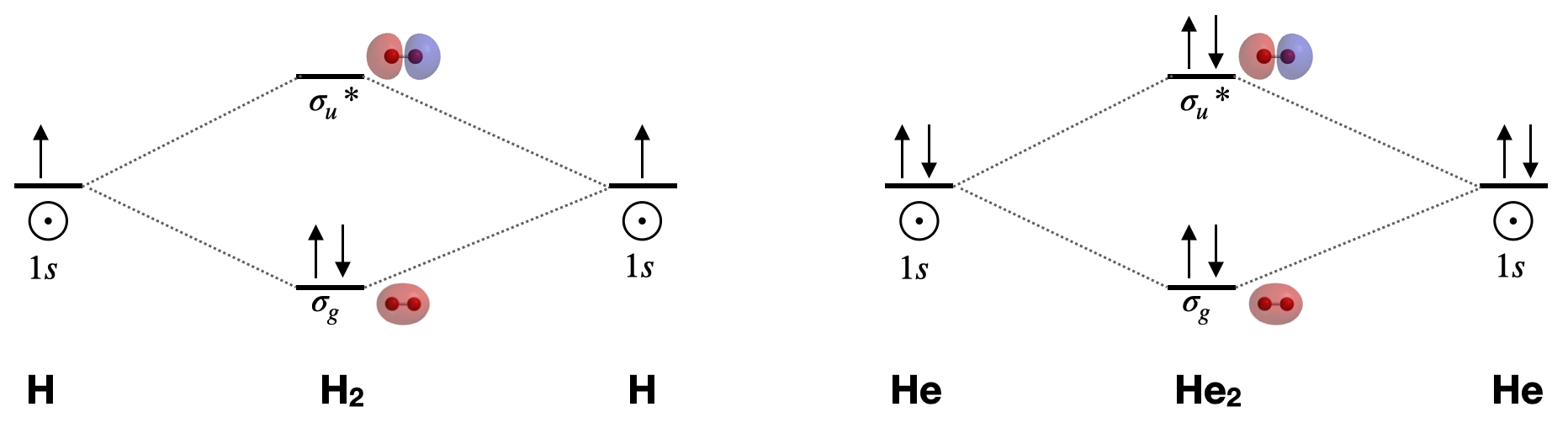
A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ...
energy molecular orbital (σ*) will be empty (recall the Aufbau Principle). While there are only two ... There would be four electrons to fill into our molecular orbital diagram and that would force us to fill in the bonding sigma MO and the anti-bonding sigma-star ... expected for a diatomic helium molecule. 3 Lecture 2 node = zero electron ...
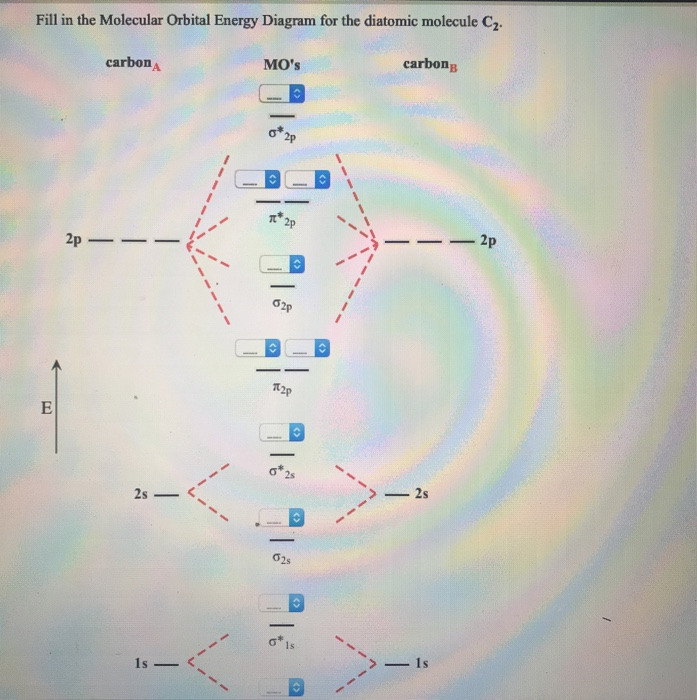
Chemistry questions and answers. Use the References to access important values Fill in the Molecular Orbital Energy Diagram for the diatomic molecule C2. carbon MO's carbong 2p - --2p T2P E 2s - 2s 625 Is - - 1s.
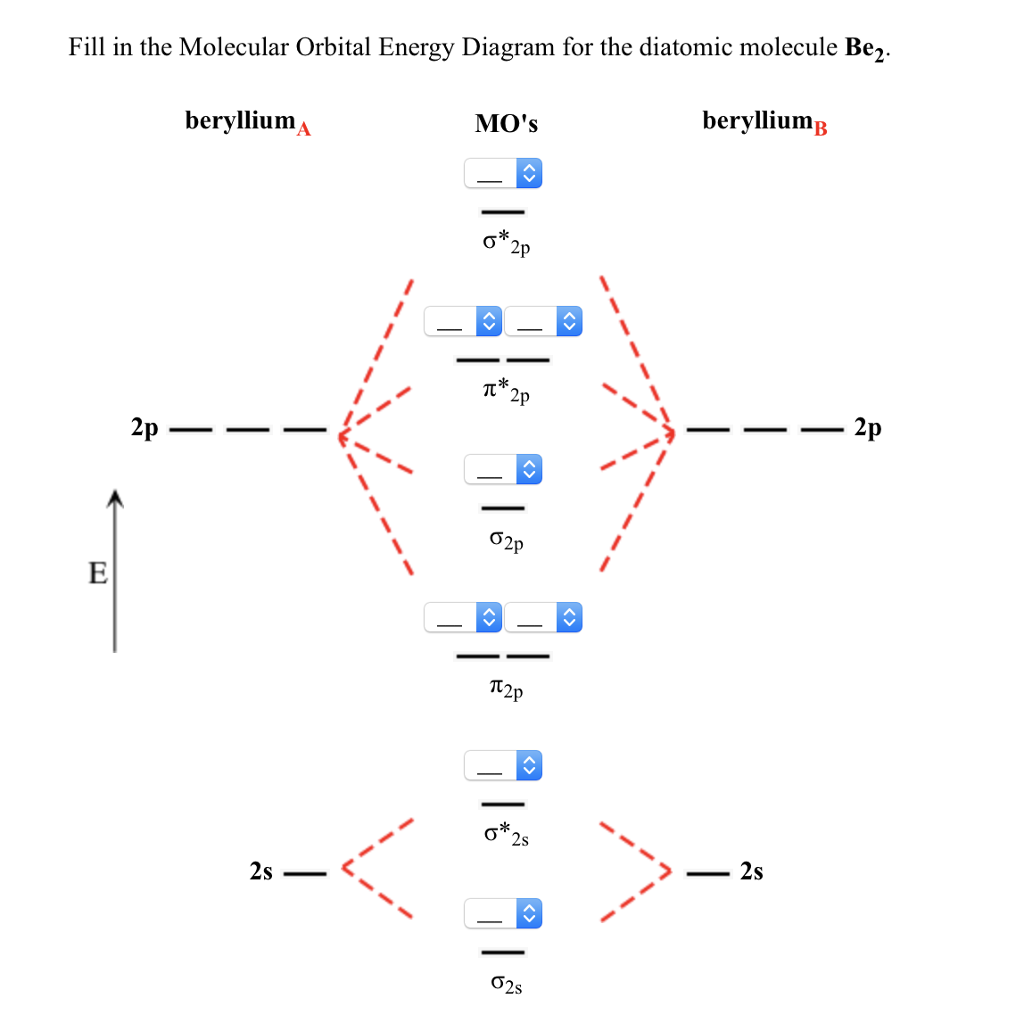
Molecular Orbital Diagrams of Diatomic Molecules Introduction: In chemistry molecular orbital (MO) theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule. The energy-level diagram for He2 is ...
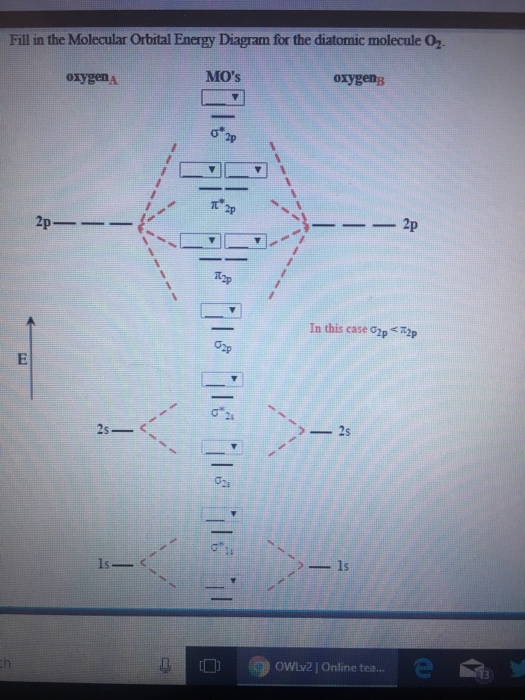
Well, s-p mixing doesn't occur with diatomic oxygen, creating a molecular orbital diagram like the first in this article. This is because, as more electrons are added to a system, the higher the energy becomes, due to their electrostatic repulsion. If the energy of the 2s and 2p orbitals are too far apart, mixing won't occur.
Molecular Orbital Diagram for a Homonuclear Diatomic • The point group for the molecule o symmetric linear molecules have D∞h symmetry o on the flow chart 1. is the molecule linear? YES 2. is there a center of inversion? YES o where does the "infinity" come from? The infinite number of possible rotation axes, Figure 1
Molecular Orbital Diagrams of Diatomic Molecules Introduction: In chemistry molecular orbital (MO) theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole molecule.
Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, "H"_2^(-) has three electrons while "H"_2^(+) has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one sigma_(1s) and one sigma_(1s)^"*" MO by conservation of orbitals.
Answer (1 of 4): Molecular orbital confuguration of Ne2 is σ1s²σ*1s²σ2s²σ*2s²σ2Pz²π2Px²π2Py²π*2Px²π*2Py²σ*2Pz² Hence the bond order of Ne2 according to M.O.T is =(Nb-Na)/2 =(10-10)/2 (since,both bonding orbitals and non-bonding orbital contains 10 electrons) =0 Hence, no bond is possible b...
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
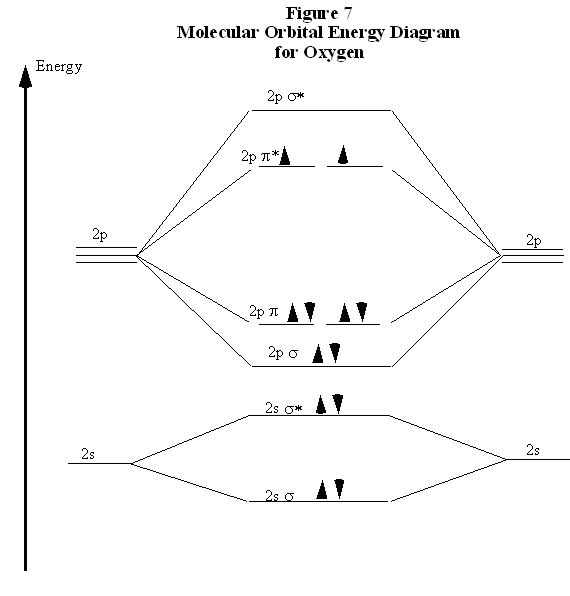
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
Printable O2 molecular orbital diagrams are available for you to guide your study in the molecular orbital lesson.This diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of a molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.
The molecular orbital energy- level diagram that results is constructed by putting the molecular orbitals in order of increasing number of internuclear nodal planes, the orbital with no such nodal plane lying at lowest energy and the orbital with nodal planes between all the atoms lying at highest energy.
C2 Molecular Orbital Diagram. The molecular orbital diagram for an o 2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p valence orbitals. The formal bond order calculated with these orbitals and occupation numbers is 2 resulting from 6 electrons in bonding orbitals ...
Complete An Mo Energy Diagram For H2+. May 25, Electronic configuration of Homonuclear Diatomic Molecules. 1)H2+. Molecular orbital energy level for H2+. The electronic configuration of H2+. Answer to Create an MO diagram for H2+ H2 and H Post the Lumo, lumo -, homo, homo + near its energy level. Molecular Orbital (MO) Theory of the H2 molecule ...
Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (13 ratings) Transcribed image text: Fill in the Molecular Orbital Energy Diagram for the diatomic molecule O2 oygenA MO's oxygeng 2p In this case ?2p 2 OWlv2 | Online tea...








0 Response to "37 fill in the molecular orbital energy diagram for the diatomic molecule h2."
Post a Comment