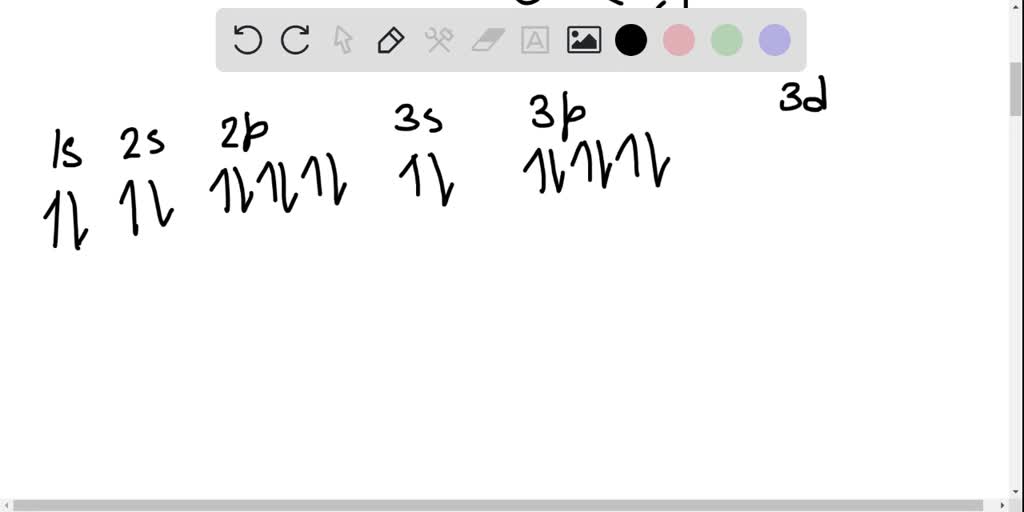
42 orbital diagram for ge
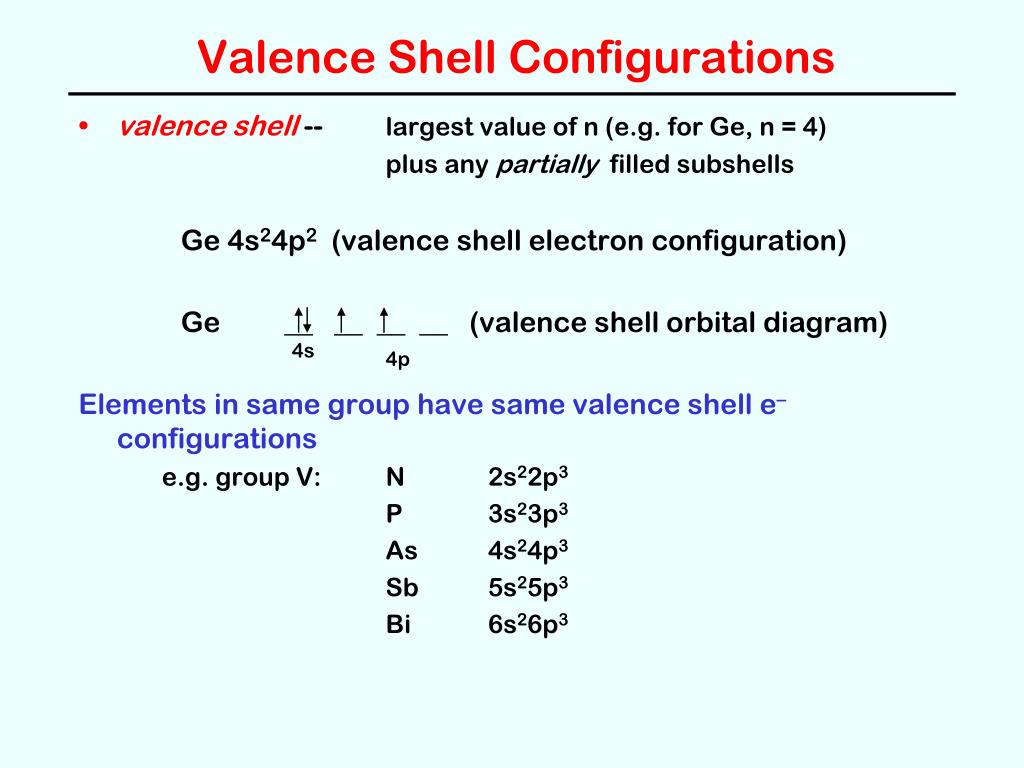
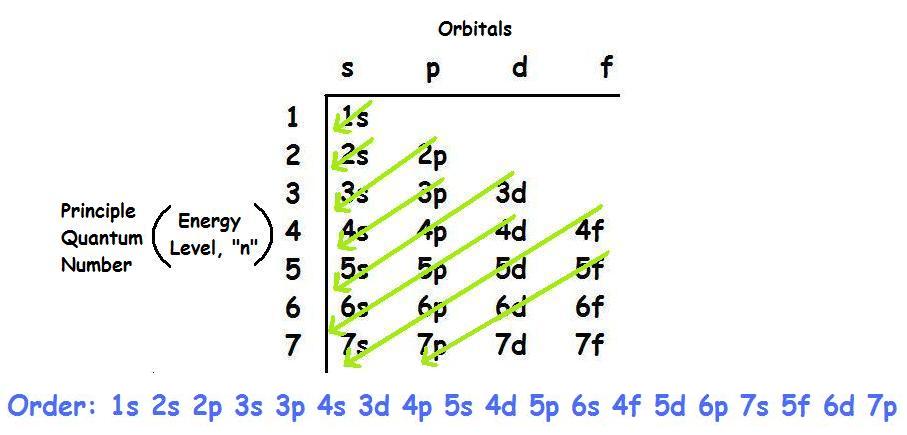
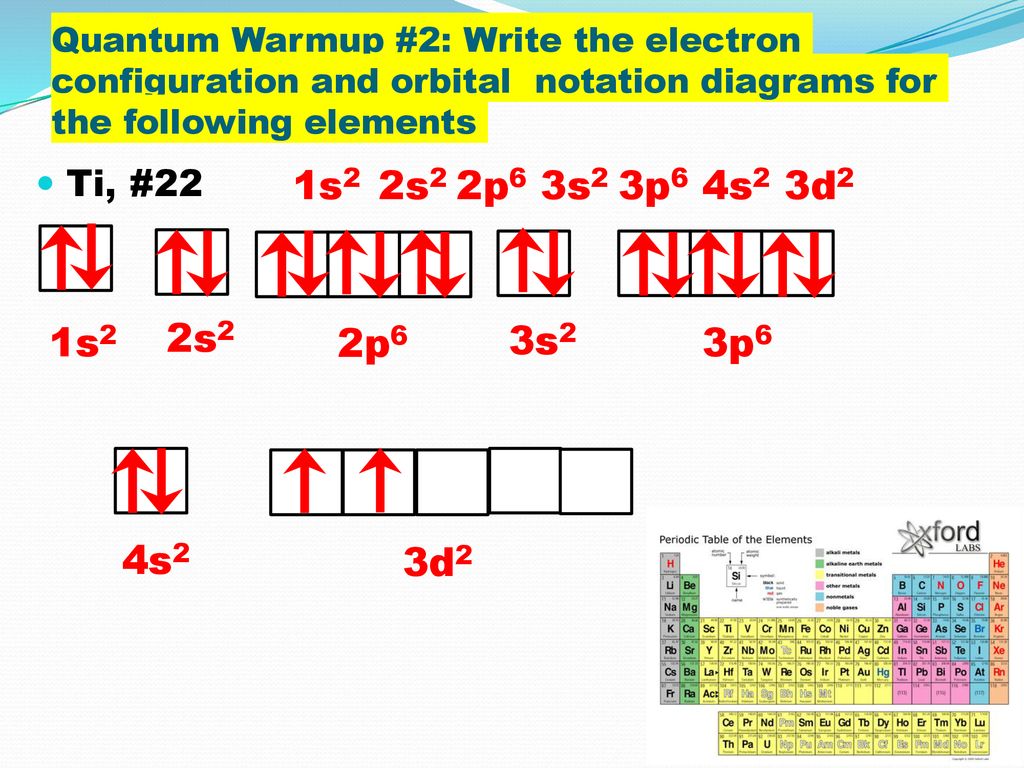
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. The above diagram roughly depicts the relative energy difference between these ... 32 Ge [Ar] 3d10 4s2 4p2 [Ar] 3d10 4s2 4p1 ... The 4s orbital is more diffuse that the 3d orbital. But the 4s orbital also penetrates to the nucleus better than the 3d orbital.
Write the electron configuration for Ge. Identify the valence electrons and the core electrons. ... the significance of orbital location, and how to represent the number of valence electrons in a ...

Orbital diagram for ge
- When comparing the two elements Ga and Ge , the element with the higher first ionization energy is Ge - When comparing the two elements P and Sb , the element with the higher first ionization energy is P ... Enter the orbital diagram for the ion Zr2+ ‣ When an element is a cation (+) you REMOVE electrons. ‣ Electrons are generally removed ... Carbon Electron Dot Diagram. If we talked about the electronic configuration of the element then, carbon is an element whose electronic configuration is given as 1s22s22p2. Now the main thing here is what electronic configuration actually means, so the solution/ answer is that in simple words, by knowing the electronic configuration of any ... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
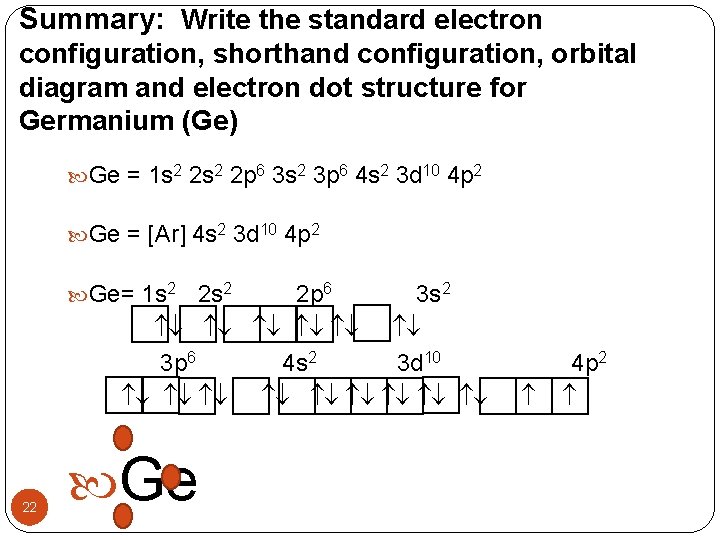
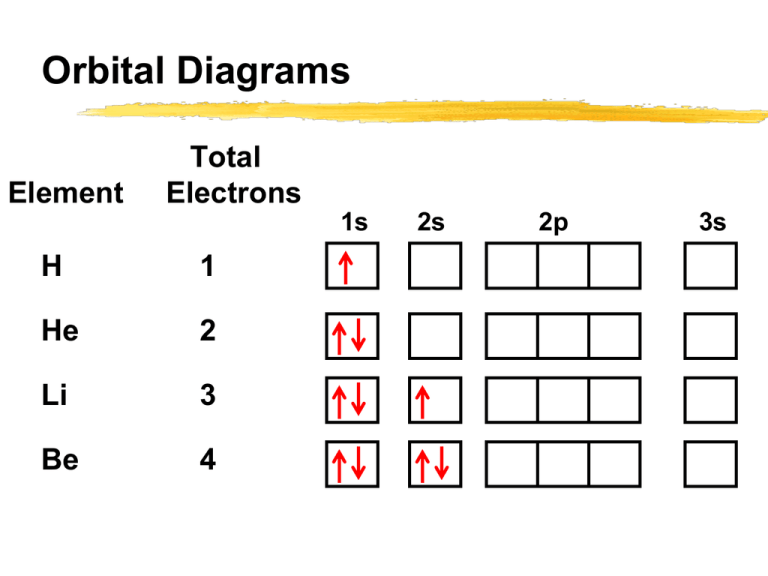
Orbital diagram for ge. 0 Comments. on Orbital Diagram For Germanium. orbital. Because an electron can have either one of two spins, any orbital can hold a maximum of four . The orbital diagram for germanium is. 1s. 2s. 2p. 3s. Oxidation States, +4,2. Electrons Per Shell, 2 8 18 4. Electron Configuration, [Ar] 3d10 4s2 4p2. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2. The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of: After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d6. Therefore the Iron electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Note that when writing the electron configuration for an atom like Fe, the 3d is usually written before the 4s. Both of the configurations have the correct numbers of ... Orbital diagrams are a visual way to show where the electrons are located within an atom. Orbital diagrams must follow 3 rules: The Aufbau principle, the Pau...
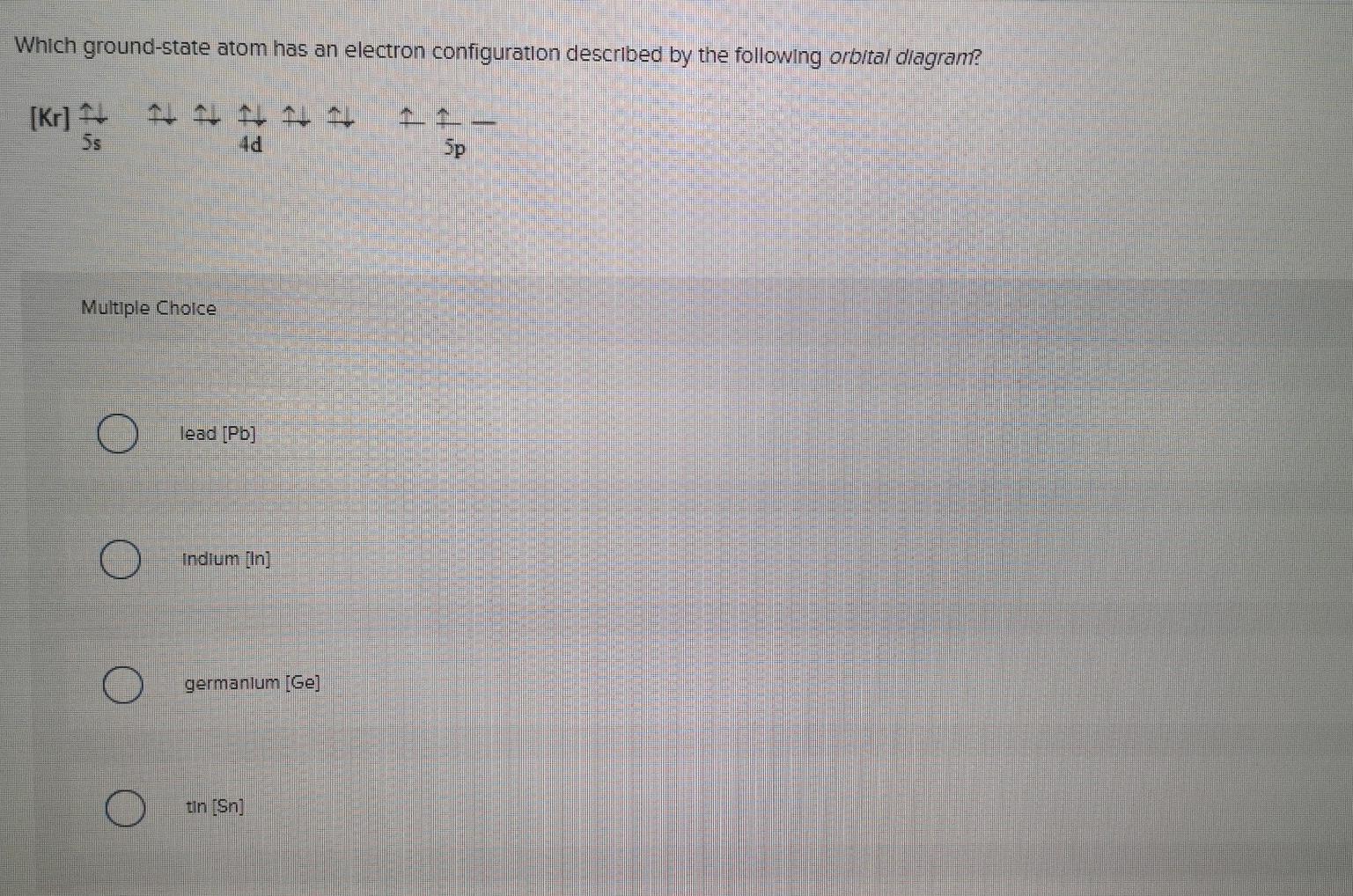
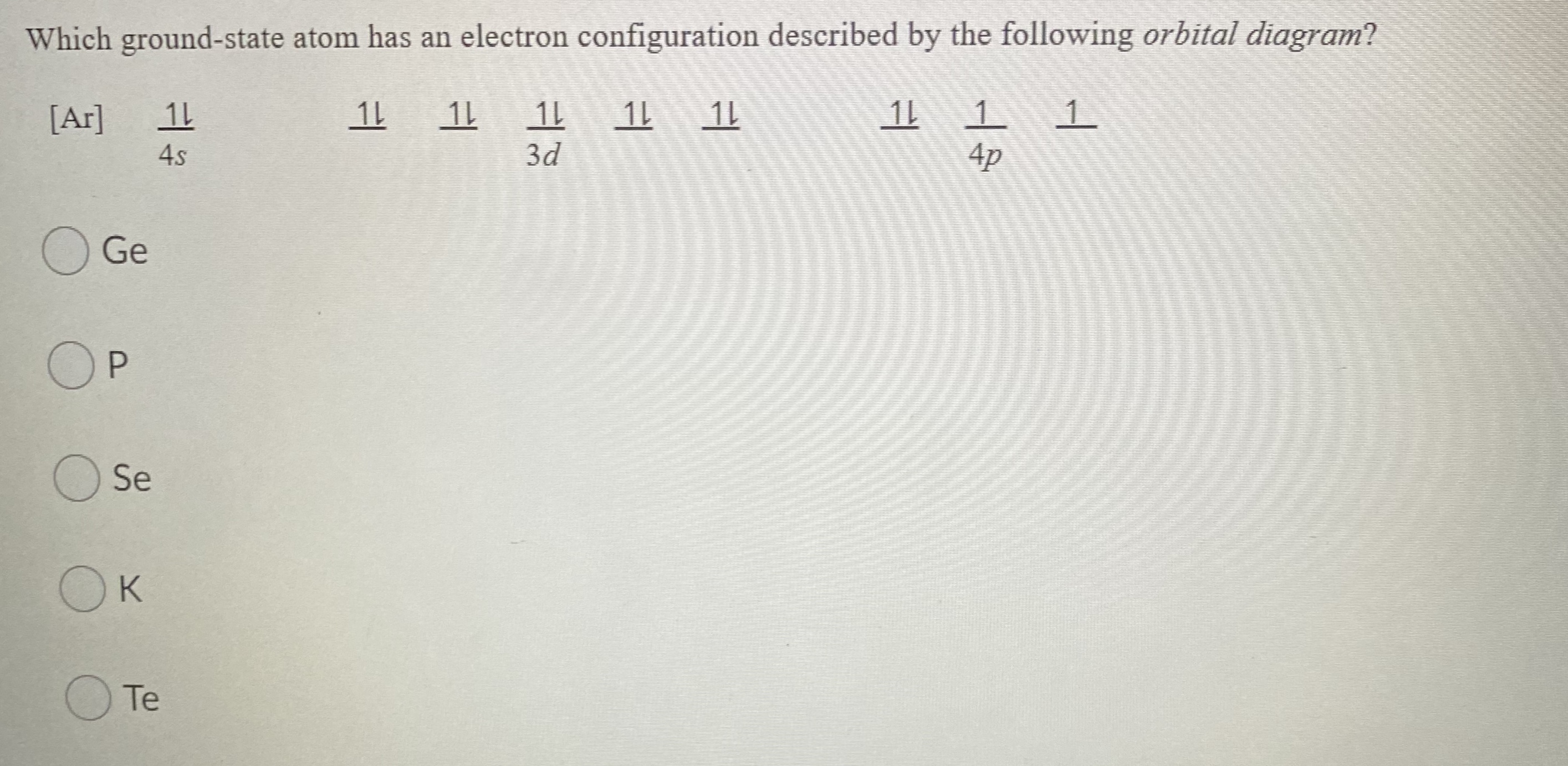
orbital diagrams •Here is the orbital diagram for Lithium (Z = 3) The shorthand convention for representing the electron configuration for Lithium is •which reads "one-ess-two, two-ess-one" 22 1s22s1 Lecture 8 - Quantum Mechanical Model of the Periodic Table The orbital diagrams can also be represented horizontally: •Build up of ... Chemistry Q&A Library What is the orbital diagram for the atom Geranium Ge 32. What is the orbital diagram for the atom Geranium Ge 32. close. Start your trial now! First week only $4.99! arrow_forward. Question. What is the orbital diagram for the atom Geranium Ge 32. check_circle Expert Answer. Orbital Filling Diagrams. An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram, the individual orbitals are shown as circles (or squares) and orbitals within a sublevel are drawn next to each other horizontally. germanium (Ge) in its ground state is A) A B) B C) C D) D E) E. C) C. Which ground-state atom has an electron configuration described by the following orbital diagram? A) phosphorus B) germanium C) selenium D) tellurium E) none of these. C) selenium. Which ground-state atom has an electron configuration described by the following orbital
the s-orbital on oxygen interacts with the in-phase combination of hydrogen, this is the lowest energy combination rather than using the p-orbital (also with a 1 symmetry) Further reading The general approach taken here was a mix between that taken in Group Theory for Chemists by Molloy, and Cottons' Chemical Applications of Group Theory . (Ge), tin (Sn), lead (Pb), and flerovium (Fl) ... electrons in the former atomic orbitals to occupy the new orbital structure without any having the same energy. Similarly if a large number N of identical atoms come together to form a solid, such as a crystal lattice, the atoms' atomic orbitals overlap.Since the Pauli exclusion principle ... 1. (30 pts) For germanium (Ge) atom: 1) Write the shorthand ground state electron configuration and orbital diagram for Ge. Indicate the magnetic property (diamagnetic or paramagnetic) of the atom. 2) Write a set of quantum numbers (n. l, m, m.) for one of the germanium valence electrons. 3) Based on the above orbital diagram, predict one ... MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
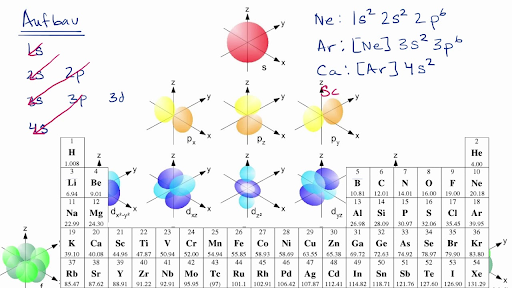
The energy of an electron in an orbital with quantum number nfor an atom with atomic number Zis given by: ... shown in the accompanying diagrams: 6d 1s 2s 3s 4s 5s 6s 2p 3p 4p 5p 6p 3d 4d 5d 4f 5f 2. Materials 100A: Orbitals, bonding, etc. 19 20 K Ca 1 2 H He 3 4 Li Be ... Ge 32 74.922 As 33 78.96 Se 34 35 79.904 37 85.468 87.62 Sr 38 88.906 Y ...
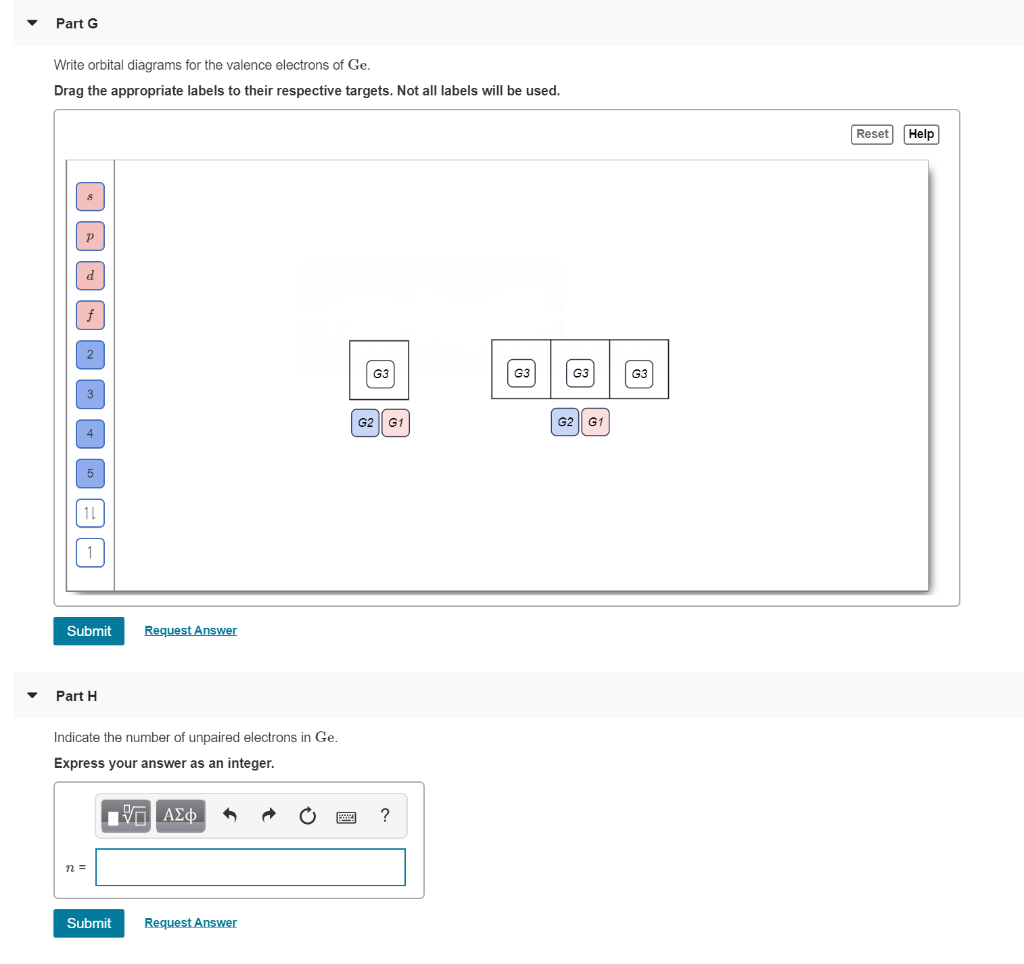
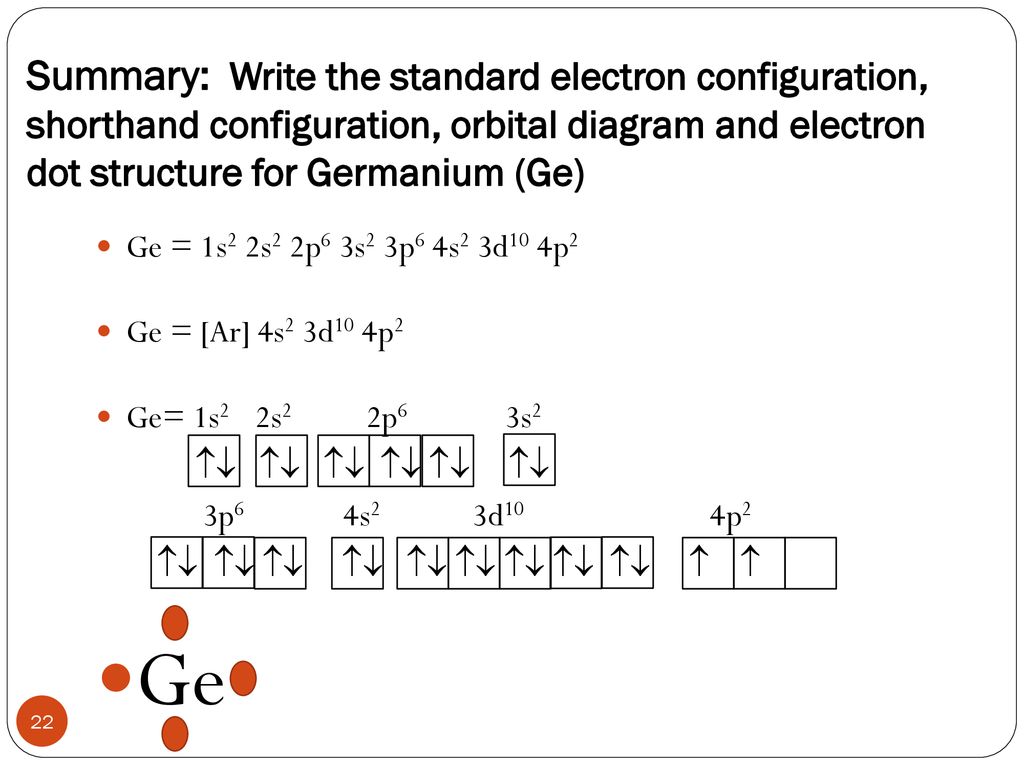
Electronic Configuration of Ge (Germanium) Atomic number of germanium is 32. Its electronic configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2 or it can be written as [Ar] 3d10 4s2 4p2. ... Orbital Diagram 1s ↿⇂ 2s ↿⇂ 2p ↿⇂ ↿⇂ ↿⇂ 3s ↿⇂ 3p ↿⇂ ↿⇂ ↿⇂ 3d ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ 4s ↿⇂ 4p ...
The fourth orbital in this subshell lies along the X and Y axes and is called the 3d x 2-y 2 orbital. Most of the space occupied by the fifth orbital lies along the Z axis and this orbital is called the 3d z 2 orbital. The number of orbitals in a shell is the square of the principal quantum number: 1 2 = 1, 2 2 = 4, 3 2 = 9.
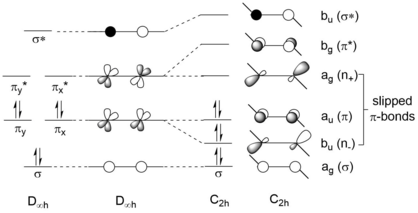
Orbital energy diagram for the frontier orbitals of MPcs (M = Ge, Sn, Pb) in the C 4v and D 4h conformation. GePc and SnPc are open-shell triplets in D 4h for which both a and b spin orbitals are ...
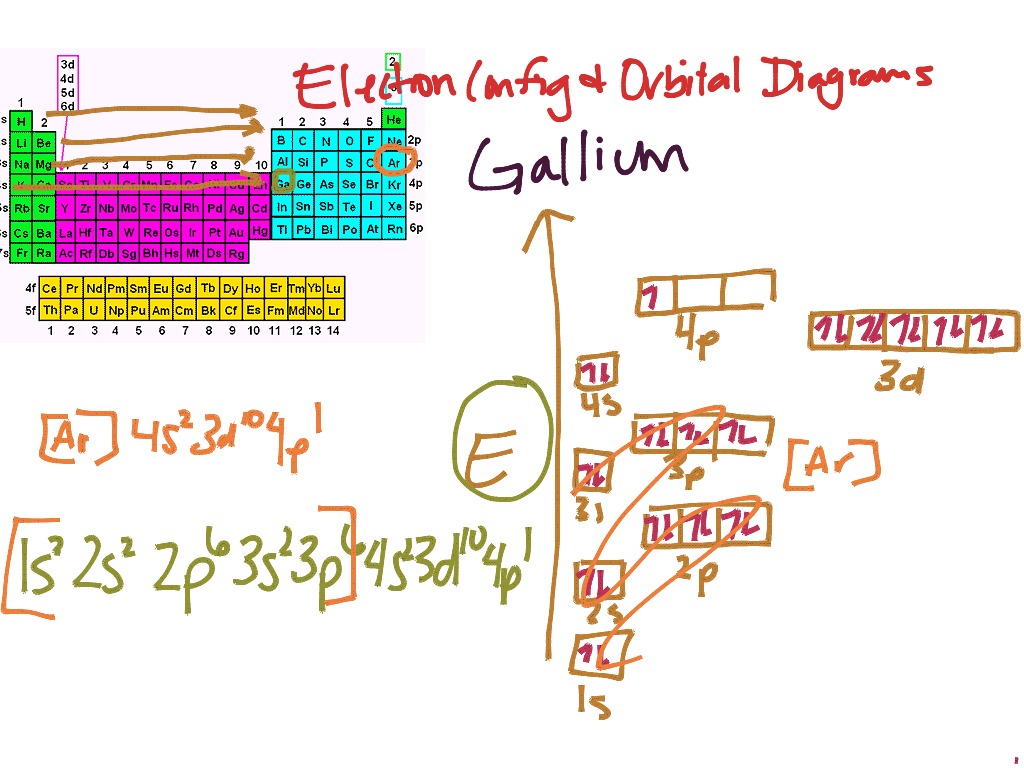
Write the electron configuration and draw an orbital diagram, showing the 3s2 3p6 4s2 3d10 4p6 5s2 4d1. 7. 1s22s22p6 3s2 3p6 4s2 3dp1. Gallium. 1s. The electron configuration for Gallium, Ga is by removing electrons first from the outermost p orbitals, followed by the s orbital and finally the d. The electron configuration for Gallium, Ga is by ...
Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium (Se) 35: Orbital diagram of Bromine (Br) 36: Orbital diagram of Krypton (Kr) 37: Orbital diagram of Rubidium (Rb) 38: Orbital diagram of Strontium (Sr) 39: Orbital diagram of Yttrium (Y) 40: Orbital diagram of Zirconium (Zr) 41: Orbital diagram ...
Germanium (Ge) has an atomic mass of 32. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
Answer (1 of 2): First of all, I hate when this happens! Your teacher is actually wrong. The electron configuration of Ge2+ is [Ar] 4s2 3d10 Below I drew out exactly what will happen when you remove (+2) 2 electrons from the Germanium ion (Ge). If you want to use a noble gas to configure an ion,...
The Electron Configuration Video Lessons. Concept: Concept: Example: Problem: Identify the element which has the following partial orbital diagram a. Ti b. Sn c. Ge d. Zr e. Pr.
Orbital Diagram Configuration Orbital Diagram Configuration . Dr. Mihelcic Honors Chemistry 2013-14 8 Homework Worksheet #7: Electron Configurations Write both the full electron configuration and draw the orbital configuration for each of the following ... Ge 5. Y 6. N . Dr. Mihelcic
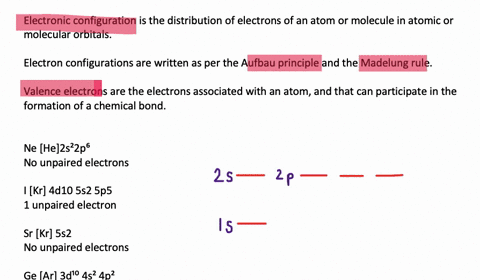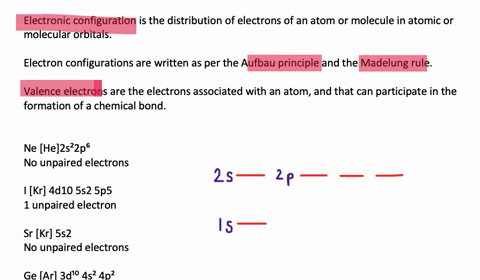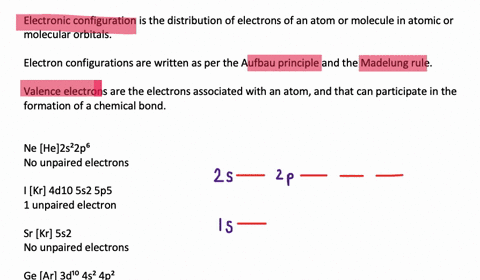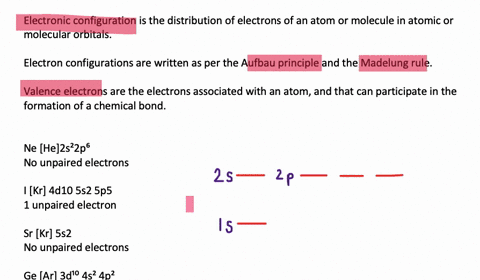
Solved Draw Orbital Diagrams Boxes With Arrows In Them To Represent The Electron Configurations Of Carbon Before And After Sp Hybridization
Germanium (Ge) is located in the fourth row, group 14 of the periodic table, and has an atomic number of 32. This implies that the neutral Ge atom's electron configuration must account for 32 electrons. So, "Ge": 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)4s^(2)3d^(10)4p^(2) An alternative way of writing the electron configuration for Ge is by using the noble gas shorthand notation.
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
Carbon Electron Dot Diagram. If we talked about the electronic configuration of the element then, carbon is an element whose electronic configuration is given as 1s22s22p2. Now the main thing here is what electronic configuration actually means, so the solution/ answer is that in simple words, by knowing the electronic configuration of any ...
- When comparing the two elements Ga and Ge , the element with the higher first ionization energy is Ge - When comparing the two elements P and Sb , the element with the higher first ionization energy is P ... Enter the orbital diagram for the ion Zr2+ ‣ When an element is a cation (+) you REMOVE electrons. ‣ Electrons are generally removed ...

Idk What I Did Wrong Interactive Figure 9 4 11 C Towards Ge Investigate Molecular Orbital Diagrams For Homeworklib






















0 Response to "42 orbital diagram for ge"
Post a Comment