42 calcium oxide electron dot diagram
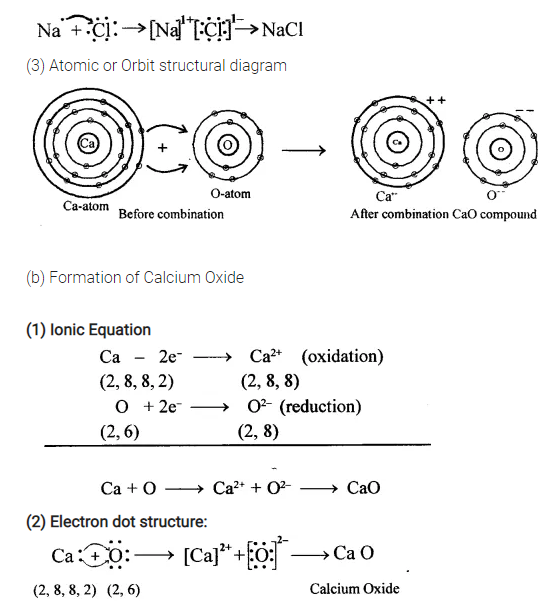
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ... When adding calcium and oxygen together, they form calcium oxide or CaO, and calciums 2 valence electrons join with oxygens 6 to fill the Lewis structure. Ca 2+ [O with 8e-] 2- Wiki User
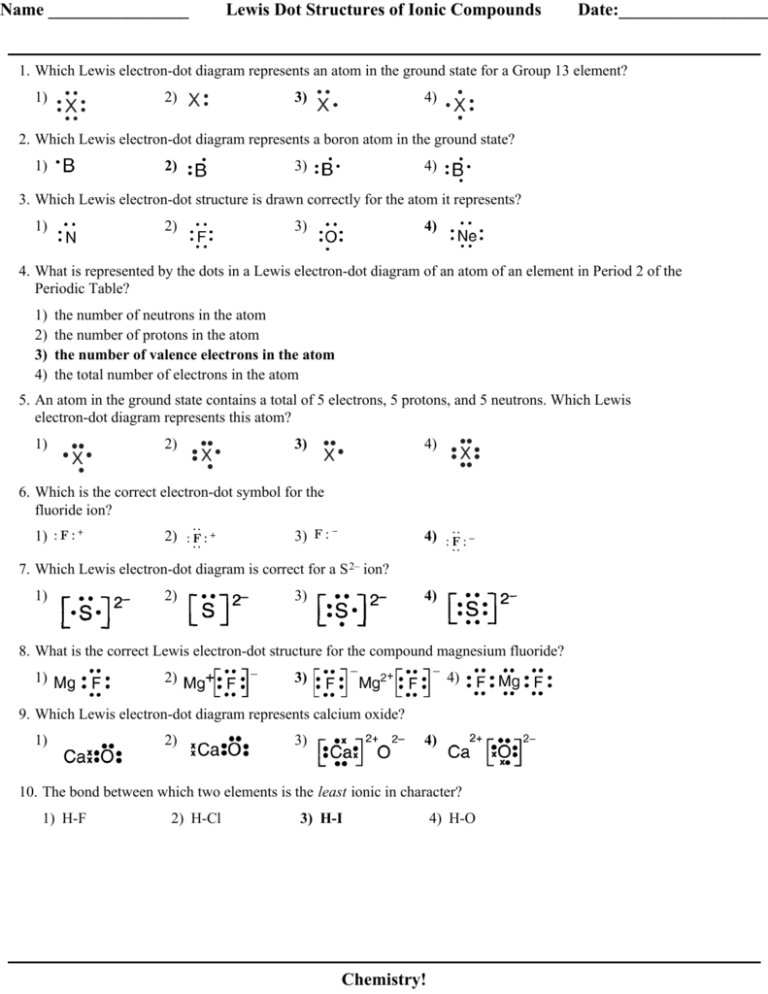
Which is the correct electron dot representation of an atom of chlorine in the ground Which element could X represent in this electron dot structure?. What is the correct formula for potassium oxide?. Calcium forms an ion with a charge of. The compound XCI is classified as ionic if X represents the element a) H b) I 14 )_Which Lewis electron ...
Calcium oxide electron dot diagram
Draw the electron-dot structure of CaO and state the type of bonding. Advertisement Remove all ads. Solution Show Solution. The electron-dot structure of CaO is: In CaO, the calcium atom transfers its two electrons to the oxygen atom to form an ionic bond. Concept: Electrovalent (or Ionic) Bond. 13 Sep 2018 — Here calcium is having an oxidation state of +2 called as cation and oxide is an anion with oxidation state of -2. Thus they combine and their ...2 answers · 113 votes: Hope it helps The valency of Ca is 2 and valency of O is 6 so Ca loses the 2 electrons and ... A step-by-step explanation of how to draw the CaO Lewis Dot Structure.For CaO we have an ionic compound and we need to take that into account when we draw th...
Calcium oxide electron dot diagram. Electron Dot Diagram study guide by Sydnee_Pollock includes 21 questions covering vocabulary, terms and more. Quizlet A model of an atom in which each dot represents a valence electron. CLICK THE Calcium, potassium, and sodium are classified under this Oxidation Number. Positive or Lewis Structure. Explanations. Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element’s symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ... 1 answerCalcium oxide (CaO) and lithium sulfide (Li2 2 S) are both ionic compounds. This is because the electronegativity difference between the... 24 Jul 2017 — (b) Show the formation of calcium oxide by the transfer of electrons. (c) Give reason for the following: (i) Ionic compounds are hard solids. ( ...
Calcium oxide electron dot diagram. The formula for silicon oxide is sio 2. When adding calcium and oxygen together they for calcium oxide or cao and calciums 2 valence electrons join with oxygens 6 to fill the lewis structure. The outer electron of the sodium atom 281 is transferred to the outer shell of the chlorine atom 287 giving it a ... Which lewis electron dot diagram represents calcium oxide show more. Well first draw. 18 22 316 418 14what is the total number of valence electrons in a sulfide ion in the ground state. Calcium oxide is an odorless white or gray white solid in the form of hard lumps. The formula for silicon oxide is sio 2. When adding calcium and oxygen together they for calcium oxide or cao and calciums 2 valence electrons join with oxygens 6 to fill the lewis structure. Calcium has 2 valence electrons while oxygen has 6 valence electrons. Lewis dot diagram represents calcium oxide. Two ca atoms for one o atom bcz ca gives off 1 electron and oxygen needs two. A step-by-step explanation of how to draw the CaO Lewis Dot Structure.For CaO we have an ionic compound and we need to take that into account when we draw th...
13 Sep 2018 — Here calcium is having an oxidation state of +2 called as cation and oxide is an anion with oxidation state of -2. Thus they combine and their ...2 answers · 113 votes: Hope it helps The valency of Ca is 2 and valency of O is 6 so Ca loses the 2 electrons and ... Draw the electron-dot structure of CaO and state the type of bonding. Advertisement Remove all ads. Solution Show Solution. The electron-dot structure of CaO is: In CaO, the calcium atom transfers its two electrons to the oxygen atom to form an ionic bond. Concept: Electrovalent (or Ionic) Bond.
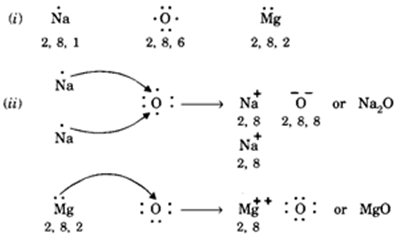
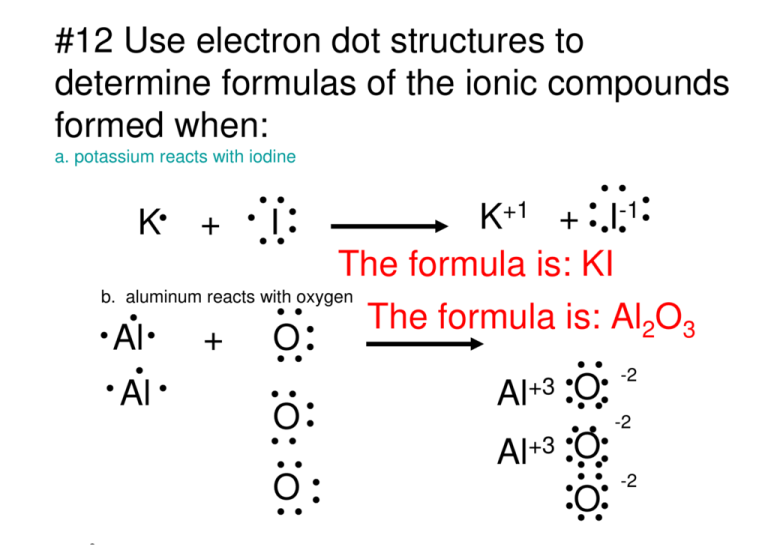
I Write The Electron Dot Structures For Sodium Oxygen And Magnesium Ii Show The Formation Of Na2o And Mgo By The Transfer Of Electrons Iii What Are The Ions Present In These Compounds From Science

Dot And Cross Diagram Of Calcium Oxide Showing The Electrons In The Outermost Shells In 2021 Electrons Diagram Chemistry
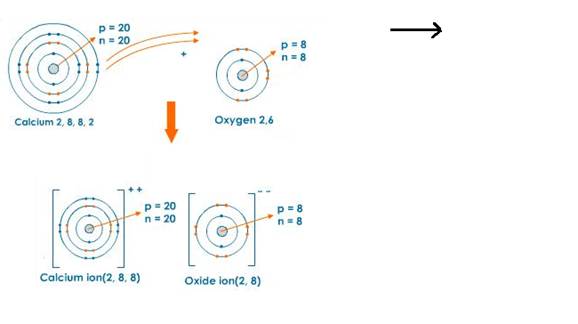
Chapter 2 Chemical Bonding Concise Chemistry Part Ii Selina Solutions For Class 10 Chemistry Icse Topperlearning
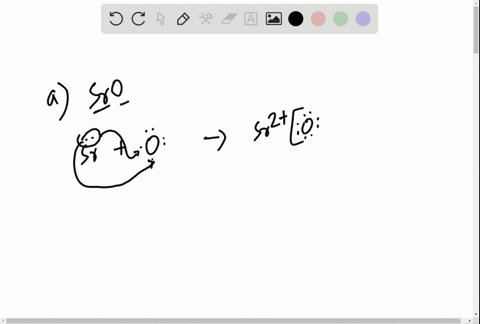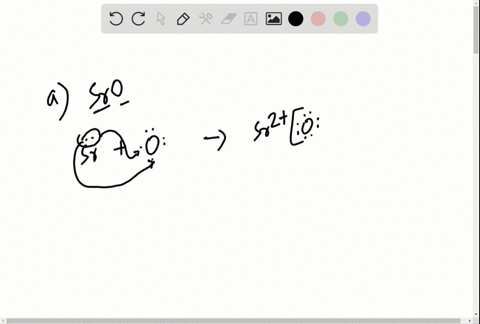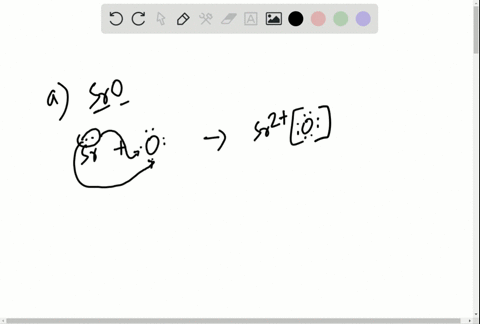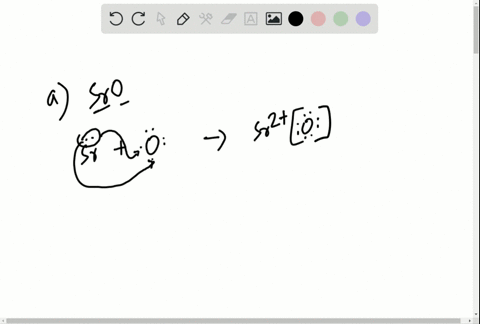
Solved Write The Lewis Symbols That Represent The Ions In Each Ionic Compound A Naf B Cao C Srbr2 D K2o

Question 1 I Write The Electron Dot Structures For Sodium Oxygen And Magnesium Ii Show The Formation Of Na2o And Mgo By The Transfer Of Electrons Iii What Are The Ions Present In

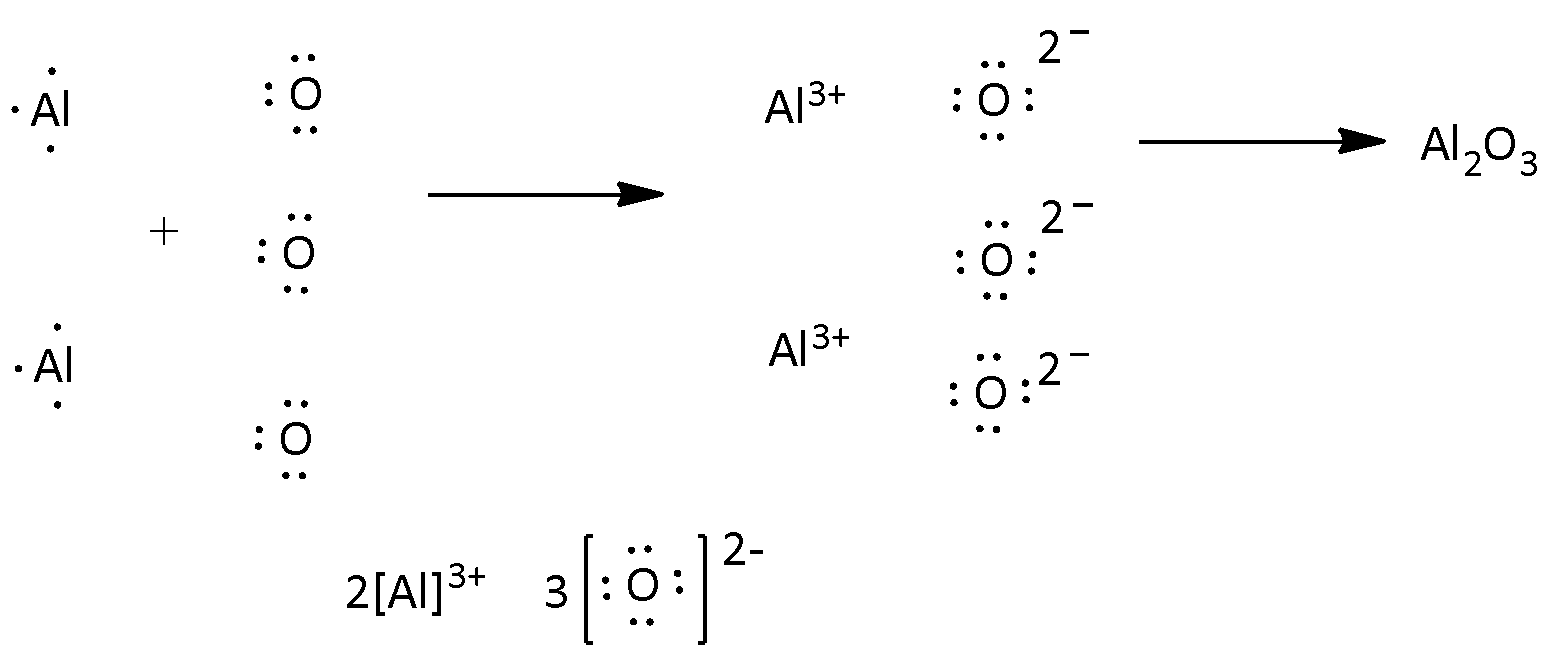
What Is The Electron Dot Structure Of Aluminium Oxide And Calcium Nitrate Science Metals And Non Metals 13062881 Meritnation Com

Lewis Structure Calcium Electron Chemistry Diagram Png Clipart Atom Black And White Brand Calcium Calcium Oxide

Explain The Formation Of Calcium Oxide By Transfer Of Electrons Step By Step Science Metals And Non Metals 14204787 Meritnation Com
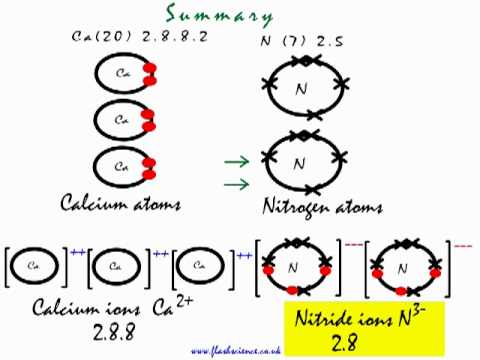


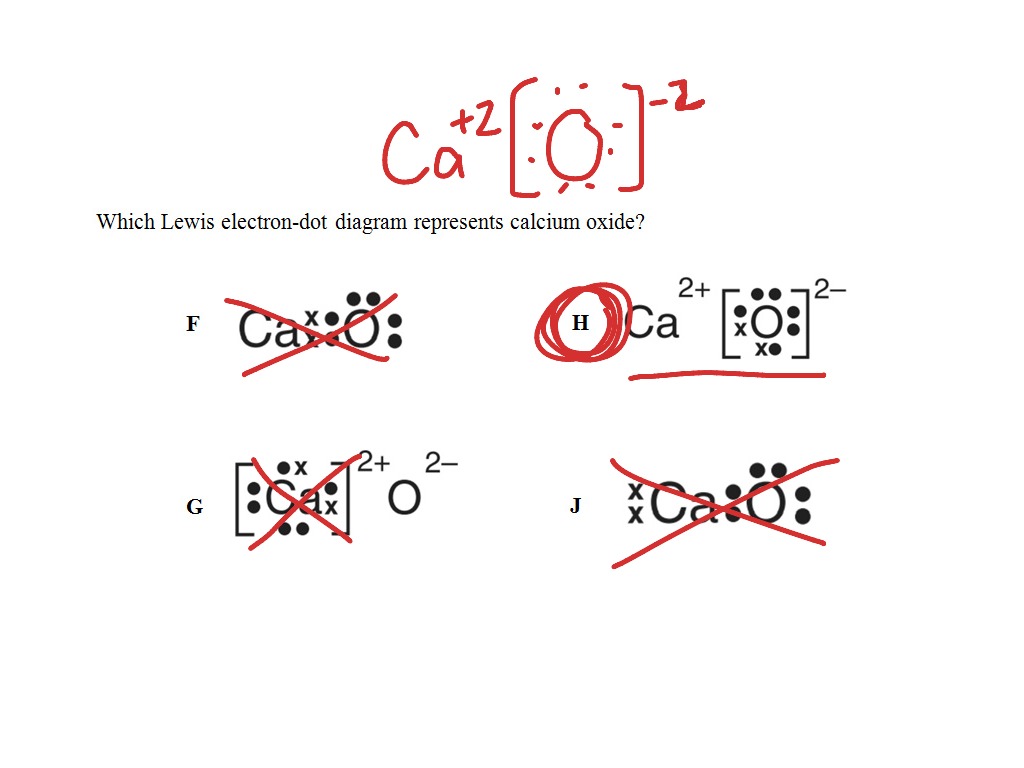

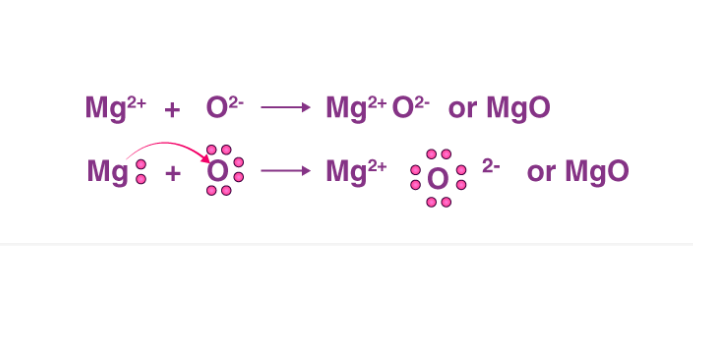
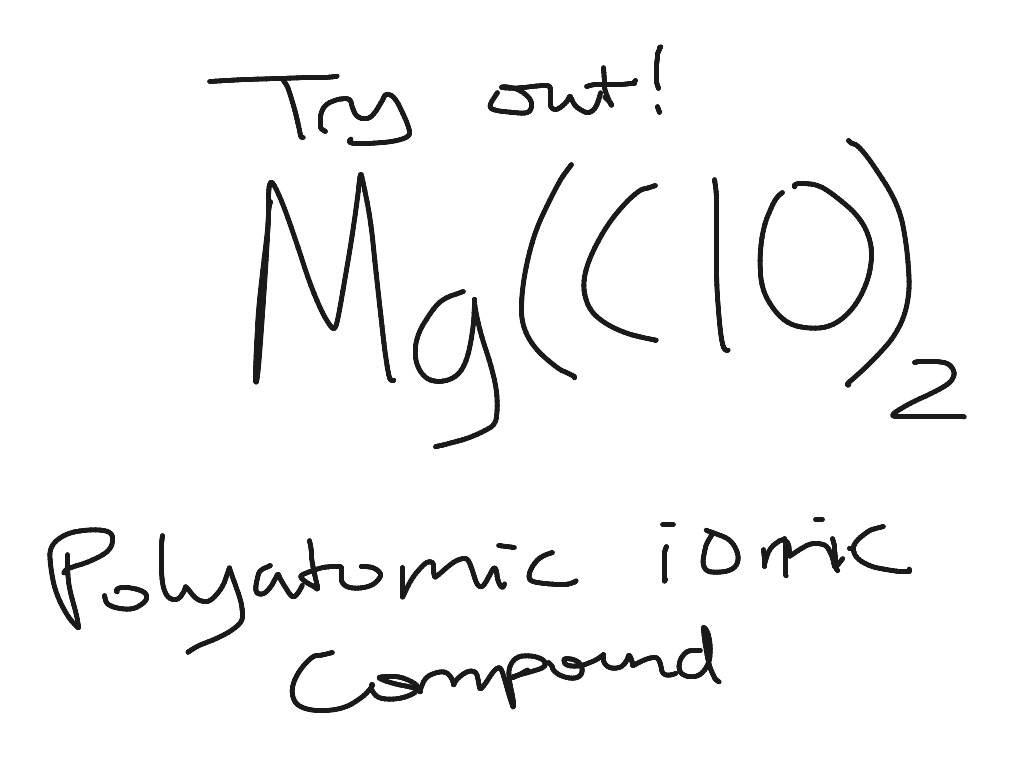









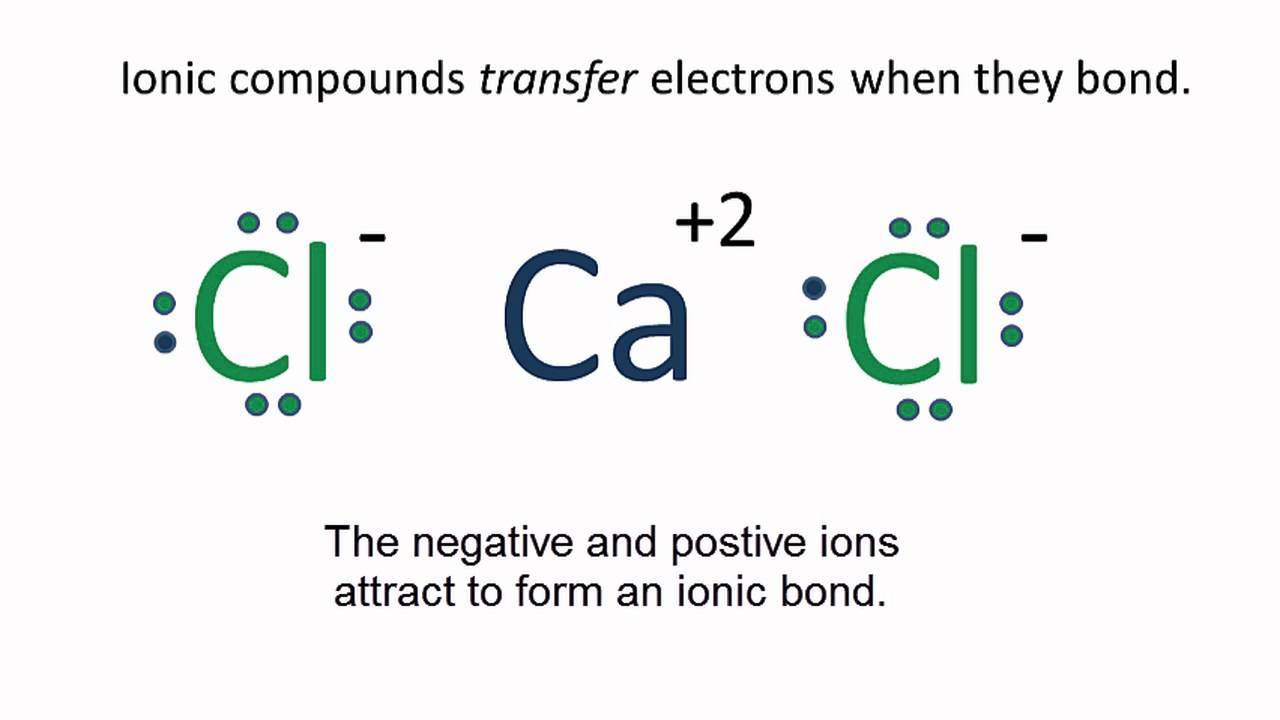
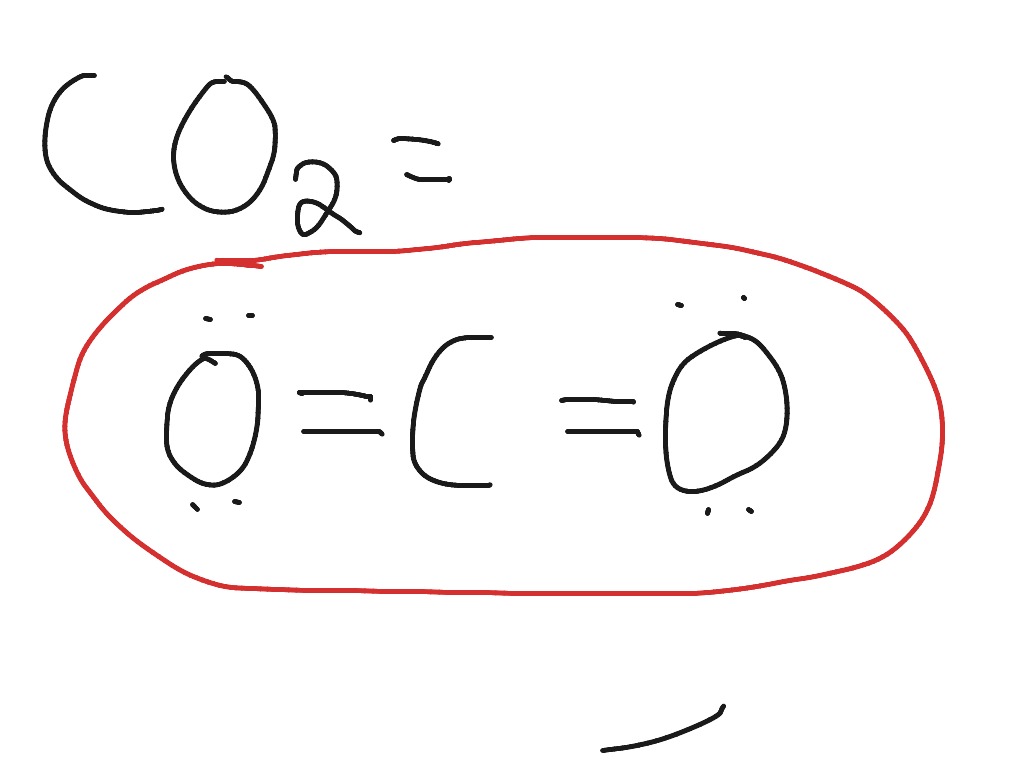



0 Response to "42 calcium oxide electron dot diagram"
Post a Comment