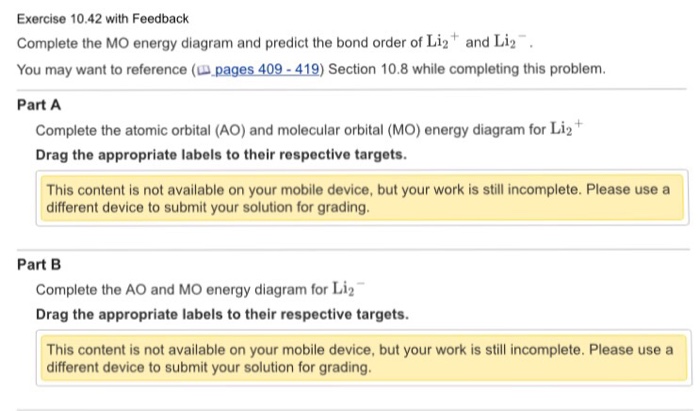
38 use the drawing of the mo energy diagram to predict the bond order of li2+.
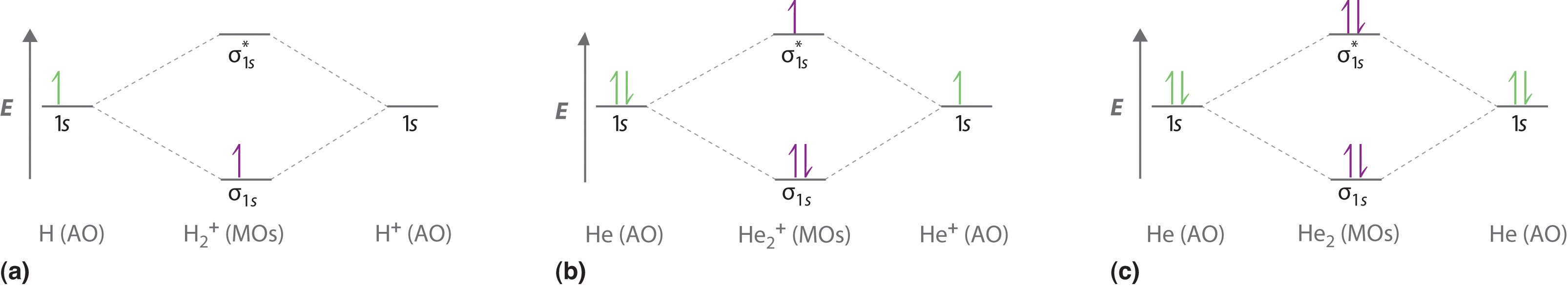
3 Aug 2018 — Here we consider the molecular orbital diagram (MO) of Li2 : ... a bonding MO, it adds 0.5 to the bond order, because more bonding character ...1 answer · No... it's the other way around. Li2 is more stable than Li+2, because the bond is (hypothetically) stronger (probably gas-phase). Here we consider ... MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
The bond order tells us the average number of bonds between the bonded atoms. In a diatomic molecule such as `O_2`, the bond order simply tells the number of bonds between the two atoms. The bond order can be interpreted from MO diagrams using the following formula: `"Bond Order" = 1/2 [("Bonding "e^-)-("Antibonding " e^-)]`
Use the drawing of the mo energy diagram to predict the bond order of li2+.
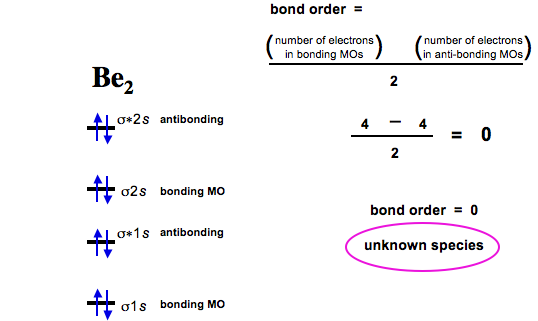
Problem: Use the drawing of MO energy diagram to predict the bond order of Be2+ and Be2–. Do you expect Be2– to exist in the gas phase? · FREE Expert Solution.1 answer · Top answer: Bond Order=12[# of e- in bonding MO-# of e- in antibonding MO]bonding MOs → without an asterisk (e.g., σ1s)antibonding MOs → those with an asterisk ... Answer to: Use the drawing of MO energy diagram to predict the bond order of L i + 2 and L i 2 . 1. Do you expect L i + 2 to exist in the... Use the drawing of the mo energy diagram to predict the bond order of li2. For the ion li2. Express your answer using two significant figures. Bond order is the number of chemical bonds between a pair of atoms. D write the electron configuration of the ion. Use the drawing of mo energy diagram to predict the bond order of be2 and be2. Do you expect these molecules to exist in the gas phase. 4 ...
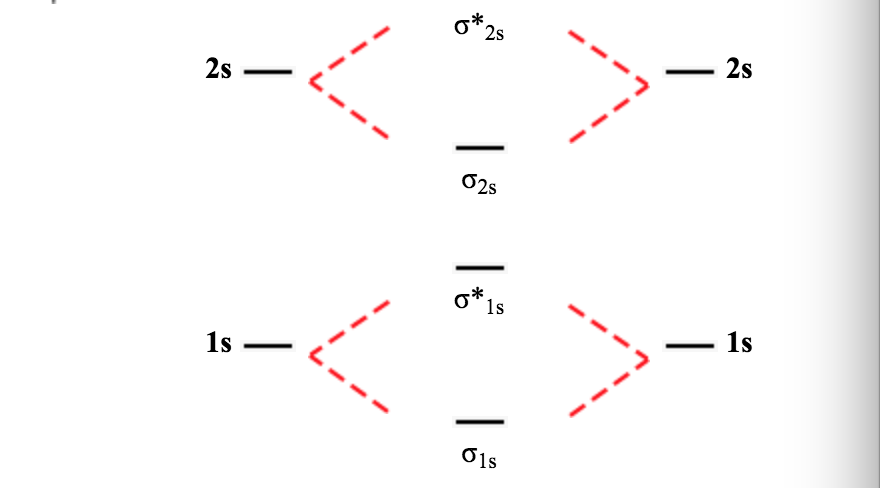
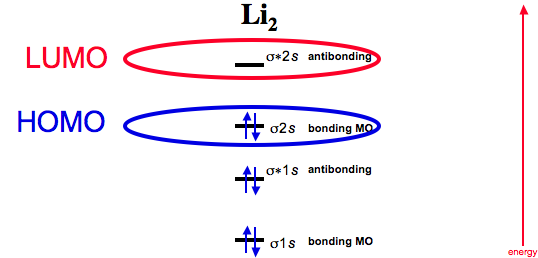
Use the drawing of the mo energy diagram to predict the bond order of li2+.. Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the energetically-favored configuration. Molecular orbital energy level of Li2. diagram; the MOs go between them. Consider the MO diagram for Li2. This is a bond between two lithium atoms, which have an electron configuration of. The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital. Using the molecular orbital energy ordering for second-period homonuclear diatomic molecules in which the p2p orbitals lie at higher energy than the s2p, draw MO energy diagrams and predict the bond order in a molecule or ion with each number of total valence electrons. Will the molecule or ion be diamagnetic or paramagnetic? a. 10 b. 12 c. 13 ... Use the drawing of the mo energy diagram to predict the bond order of li2. Draw an molecular orbital energy diagram and predict the bond order of be2 and be2. Part a complete the mo energy diagram for the n2 ion by dragging the electrons electron with spin up in the figure given below.
Solution for Use the drawing of MO energy diagram for CO to predict the bond order. (Use the energy ordering of O2. ) Do you expect these molecules to exist in the gas phase? Answer. See drawing. Since both have a bond order of + ...7 Nov 2019 Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. Use the drawing of the MO energy diagram to predict the bond order of Li2?. Express the bond order as an integer or fraction. Part C. Which molecules are predicted to exist in the gas phase? Check all that apply This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be . Learn to draw molecular orbital electron configuration energy diagrams. molecular orbital electron configuration diagram for Li2 (Figure "Molecular orbital.
the bond order is negative or zero. 3) Relative stability of molecule in terms of bond order. For diatomic molecules ,the stability is directly proportional to the bond order. A molecule with the bond order of 3 is more stable than a molecule with bond order of 2 and so on. 4) Nature of bond in terms of bond order : Bond order 1 ,2 and 3 mean ... As discussed in class the MO diagram for B 2 shows that it has two unpaired electrons (which makes it paramagnetic) and these electrons are in bonding molecular orbitals resulting in the equivalent bond strength of one bond. As discussed in class it is not a bond. This example was covered in class to show the rare exception that this single bond is a bond. Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion 1 Order of filling of molecular orbitals in heteronuclear diatomic molecules such as CO. Part A. Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. Problem: Draw the MO energy diagram for HCl on your own, then use it to predict the bond order for the molecule. FREE Expert Solution. 81% (79 ratings) FREE Expert Solution. First, identify the number of valence electrons. The number of valence electron per element is based on the group number.
How does bond order correspond to phase? Use the drawing of MO energy diagram to predict the bond order of [Be2]+ and [Be2]−. Determined that the bond order of [Be2]+ is (+1/2).
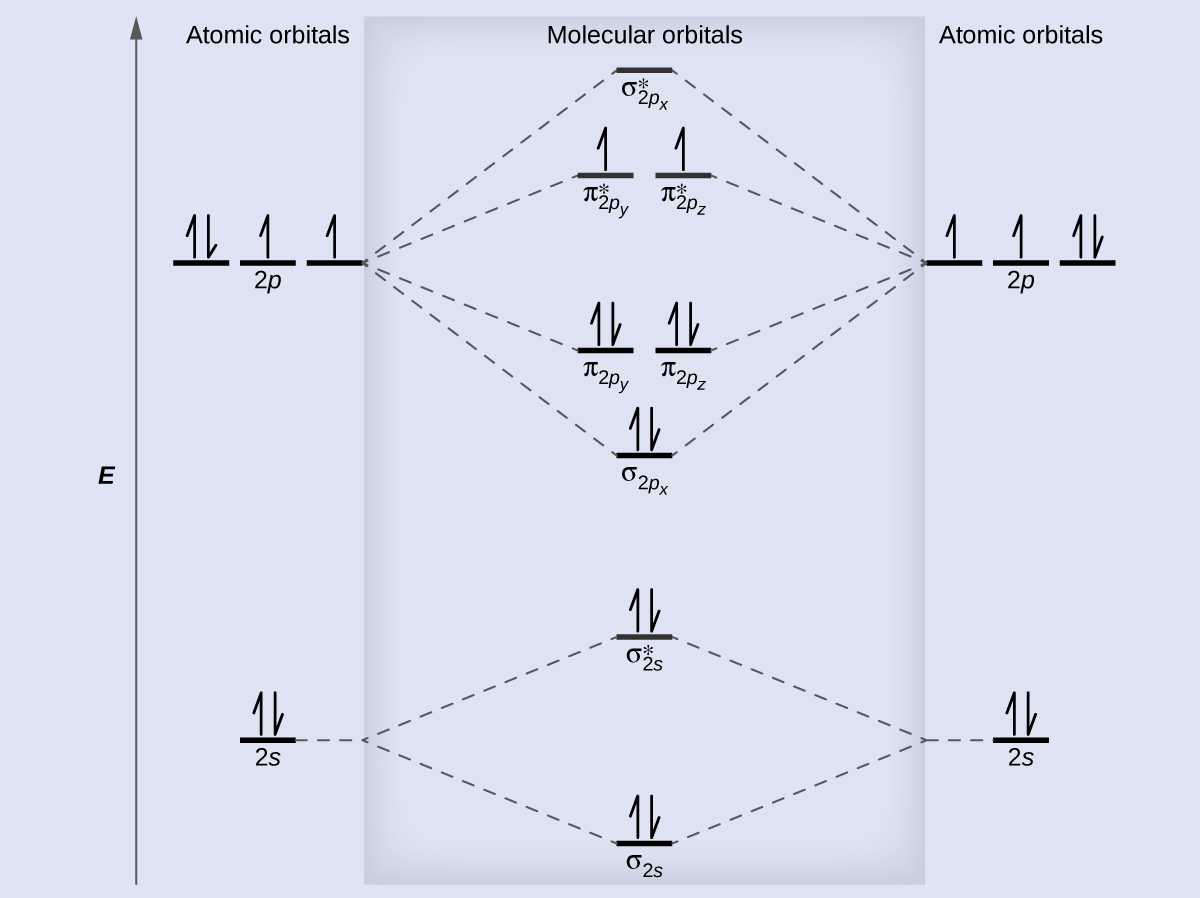
bond orderthe number of overlapping electron pairs between a pair of atoms. antibondingan atomic or molecular orbital whose energy increases as its constituent atoms converge, generating a repulsive force that hinders bonding. Bond order is the number of chemical bonds between a pair of atoms; in diatomic nitrogen (N≡N) for example, the bond ...
Learn how to apply molecular orbital theory to determine the shapes of bonded orbitals, recognize molecular orbital diagrams, calculate bond order, and ...1 answer · Top answer: The MO diagram for both Li+2Li2+ and Li2Li2 are shown below. • Li+2Li2+ has only 1 valence electron so the only...
Use the drawing of MO energy diagram for CO to predict the bond order. (Use the energy ordering of O2) The two sigma bonds form between a hybrid sp orbital on Be and a p orbital on Br. Indicate which orbitals overlap to form the σ bonds in the following molecules: BeBr2.
Be2 has a bond order of 12 and for be2 it has a bond order of 0. Draw an molecular orbital energy diagram and predict the bond order of li2 and li2. Interviews 1 revell k. Use the drawing of mo energy diagram to predict the bond order of be2 and be2. Express your answer using two significant figures. D write the electron configuration of the ion.

Use The Drawing Of Mo Energy Diagram To Predict The Bond Order Of L I 2 And L I 2 1 Do You Expect L I 2 To Exist
In the formation of B 2 molecule, three valence electrons of each boron atom i.e. 6 in all, have to be accommodated in various molecular orbitals in the increasing order of their energies. MO electronic configuration: Bond order: Here Nb = 4, Na = 2 Bond order = The two boron atom is B2 molecules are linked by one covalent bond.
Draw the MO diagram for acetylide ion C2^2- and calculate its bond order.
Mo for li2 is given below. Molecular orbital diagrams for li2 li2 be2 b2 c2 n2. Calculate the number of bonding and antibonding electrons in simple molecules. For the second period elements the 2 s and 2 p orbitals are important for mo considerations. Draw a molecular orbital energy diagram and predict the bond order of li2 and li2.
17:57Also explain how to calculate the bond order for Li2 Li2+ Li2 - . ... ,+ Li2- Molecular orbital diagram draw ...13 Jun 2020 · Uploaded by Digital Kemistry
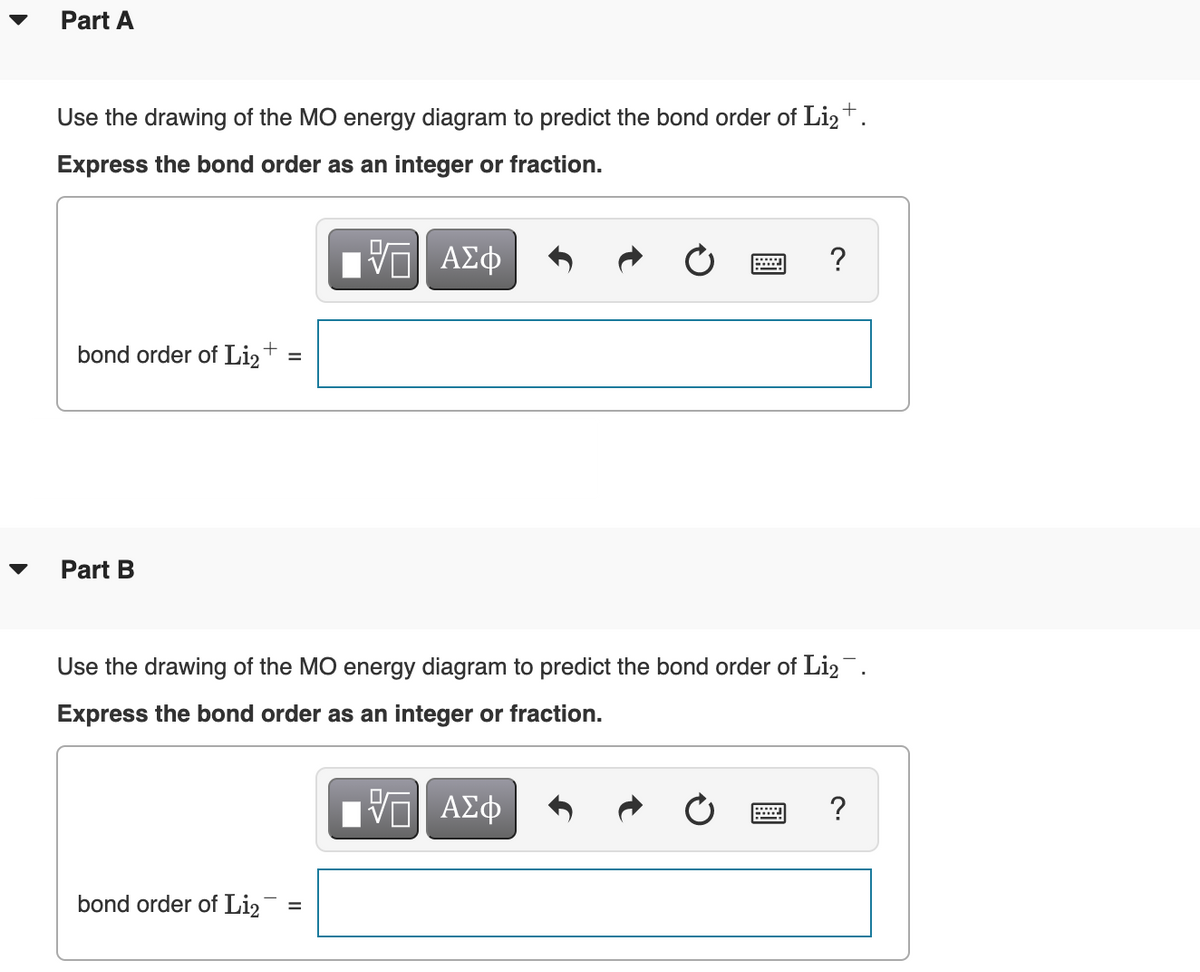
Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its positive and negative ions should be stable. Part A. Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. Use the ...
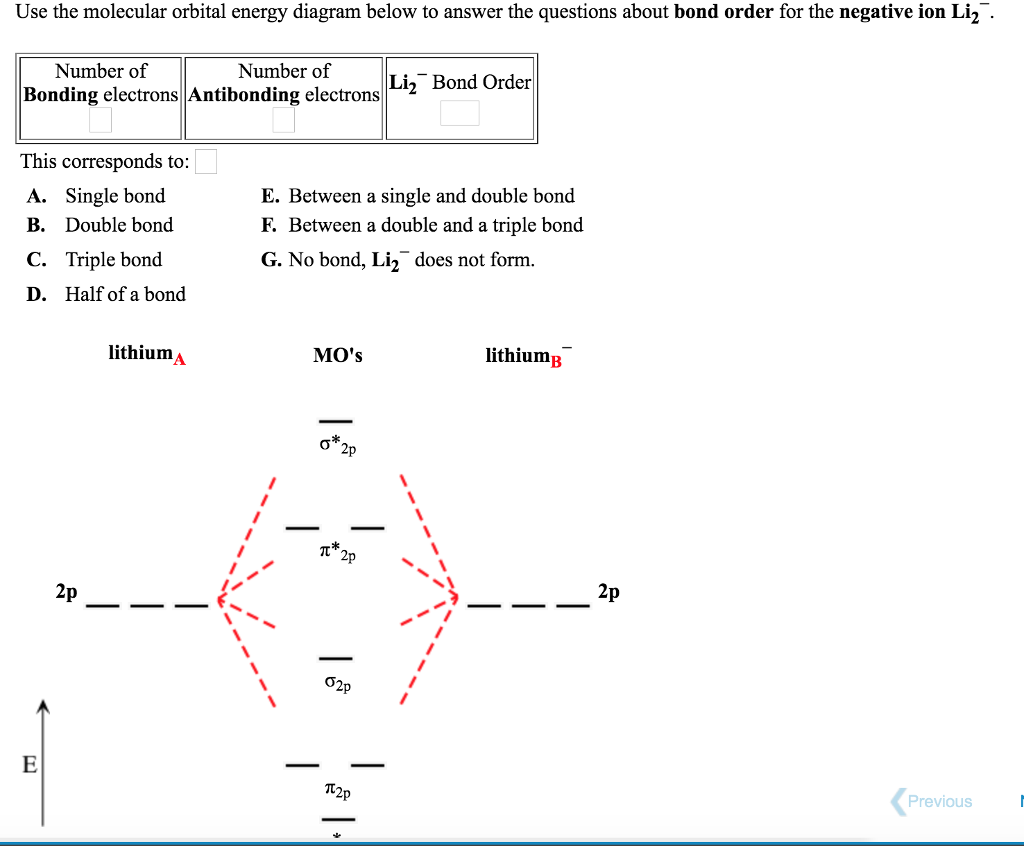
Express the bond order as an integer or fraction. Use the drawing of the MO energy diagram to predict the bond order of Li2−. Which molecules are predicted to exist in the gas phase?
Solved Draw An Mo Energy Diagram And Predict The Bond Order Of Be 2 And Be 2 Do You Expect These Molecules To Exist In The Gas Phase Course Hero
Draw an molecular orbital energy diagram and predict the bond order of li2 and li2. Use the drawing of mo energy diagram to predict the bond order ofli2 and li2. Part a complete the mo energy diagram for the n2 ion by dragging the electrons electron with spin up in the figure given below.
Use the molecular orbital diagram shown to determine which of the following is paramagnetic. asked Jul 15, 2019 in Chemistry by brittanyr9777. general-chemistry. Nitrogen can lose an electron to form N2+. Given the molecular orbital configuration of N2 [core] (σ2s)2 (σ *2s)2 (π2p)4 (σ2p)2 is N2+ diamagnetic or paramagnetic? asked Jun 30 ...
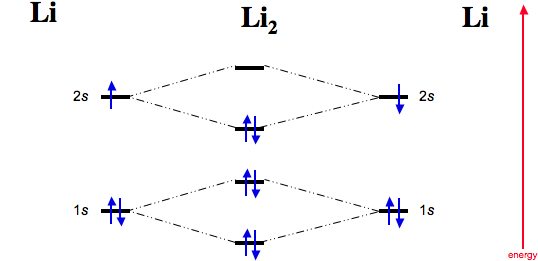
The bond order for Li2 is 1. The bonding and antibonding MOs formed from the 1s orbitals of the two atoms will have 2 electrons each. And the two electrons of the 2s orbitals will occupy the bonding MO, leaving the antibonding MO empty. Thus, there are altogether 4 bonding and 2 antibonding electrons.
Bond order is less in solid phases usually 1 intermediate in liquid 2 or 3 and greatest in gases 3. Use the drawing of the mo energy diagram to predict the bond order of li2. Bond order is the number of chemical bonds between a pair of atoms. Use the drawing of mo energy diagram to predict the bond order of be2 and be2.
Use the drawing of the mo energy diagram to predict the bond order of li2. Complete the ao and mo energy diagram for li2. Answer to 1complete the atomic orbital ao and molecular orbital mo energy diagram for li2 and li2. Each mo can hold two es and hence for li2 the mo scheme is σ σ0 li2 is present the extent of 1 in lig. Determine the magnetism of simple molecules. Use the drawing of the mo ...
Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. Use the drawing of the MO energy diagram to predict the bond order of Li2?. Express the bond order as an integer or fraction. Part C. Which molecules are predicted to exist in the gas phase? Check all that apply
Part BUse the drawing of the MO energy diagram to predict the bond order of ... Part CWhich molecules are predicted to exist in the gas phase?A. Li2B. Li2+ ...1 answer · Top answer: We are being asked to draw the MO energy diagram of Li2+ and Li2- then predict which will exist in the gas phase.We will do the following stepsStep 1: Calculate ...

How To Draw Molecular Orbital Diagram Of Li2 Li 2 Li2 Simplest Trick Chemistry Best Online Free Chemistry Class 9 12
Use the drawing of the mo energy diagram to predict the bond order of li2. For the ion li2. Express your answer using two significant figures. Bond order is the number of chemical bonds between a pair of atoms. D write the electron configuration of the ion. Use the drawing of mo energy diagram to predict the bond order of be2 and be2. Do you expect these molecules to exist in the gas phase. 4 ...

Solved Draw An Mo Energy Diagram And Predict The Bond Order Of Li2 And Li2 Do You Expect These Molecules To Exist In The Gas Phase
Answer to: Use the drawing of MO energy diagram to predict the bond order of L i + 2 and L i 2 . 1. Do you expect L i + 2 to exist in the...
Problem: Use the drawing of MO energy diagram to predict the bond order of Be2+ and Be2–. Do you expect Be2– to exist in the gas phase? · FREE Expert Solution.1 answer · Top answer: Bond Order=12[# of e- in bonding MO-# of e- in antibonding MO]bonding MOs → without an asterisk (e.g., σ1s)antibonding MOs → those with an asterisk ...

Key Equations Chem Mos Of The Molecule Would You Expect Them To Have A Greater Atomic Orbital Contribution From C Have A Greater Atomic Orbital Contribution From X Or Be
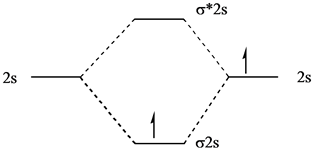
Solved Chapter 10 Problem 42e Solution Masteringchemistry Standalone Access Card For Principles Of Chemistry 2nd Edition Chegg Com












0 Response to "38 use the drawing of the mo energy diagram to predict the bond order of li2+."
Post a Comment