38 copper gold phase diagram
copper oxide is painted on the article and then gold balls are attached. The article is then placed in a reducing flame which causes the oxide to reduce to copper and then react and form a eutectic with the gold which disappears by diffusion. Several objects that are thought to have been bonded using this process This representation is called a phase diagram. The phase diagrams of some binary systems relevant to bronze show the behavior of alloying elements that typically results in one of the three cases mentioned previously. The copper-tin equilibrium phase diagram (Figure 3) illustrates Cases (1) and (2).
Abstract. This article is a compilation of binary alloy phase diagrams for which copper (Cu) is the first named element in the binary pair. The diagrams are presented with element compositions in weight percent. The atomic percent compositions are given in a secondary scale. For each binary system, a table of crystallographic data is provided ...
Copper gold phase diagram
Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash Metals like silver and gold have a difference of 0.2%; nickel and copper of 2.7%, and show complete solid solubility. But zinc and copper have 4.2% difference with maximum solubility of 38.4 wt.% Zn. (other factors are less favourable); Cadmium in copper with 16.5% size difference shows a solid solubility of 1.7 wt.%. Eutectic phase diagram for a silver-copper system. 2800 2600 2400 2200 2000 1800 1600 MgO CaO 20 40 60 80 100 0 C) L MgO ss + L MgO ss CaO ss + L CaO ss MgO ss + CaO ss Wt % Eutetic phase diagram for MgO-CaO system. Temperature (Lecture 19 - Binary phase diagrams 4 of 16 11/23/05
Copper gold phase diagram. Copper is an essential trace element in plants and animals, but not all microorganisms. The human body contains copper at a level of about 1.4 to 2.1 mg per kg of body mass. Absorption. Copper is absorbed in the gut, then transported to the liver bound to albumin. Copper in Powder Metallurgy. A. Phase Diagrams 1. Iron-Copper System The iron-copper phase diagram, taken from Hansen,3 is presented in Figure 1. Hansen3 also gives a thorough review of the work done on the system up to 1957. A review of more recent work, up to 1963 was done by . Elliott~ The most recent version of the phase diagram Journal of Phase Equilibria - Indicates key paper06Rue: R. Ruer, "Alloys of Palladium with Gold,"Z. Anorg.Chem., 51, 391-396 (1906) in German.(Equi Diagram; Experimental; Indicates presence of a phase diagram) ในระบบที่ประกอบด้วยโลหะ 2 ชนิด (Binary phase diagram) ที่มีการ ... 12 Figure 9.2 The copper-nickel phase diagram . 3. ปริมาณหรือสัดส่วนของ Phase ที่ปรากฏอยู่ (Phase Amounts) 2.
phase diagrams. Copper-gold, which forms a continuous range of fcc solid solutions at elevated temperatures, has been exploited since ancient times [6] due to its natural occurrence and attractive reddish colour. Its binary phase diagram has been intensively investigated, primarily because its high temperature α-(Au,Cu) solid solution undergoes Copper Silver Phase Diagram Phase Equilibria In The Agcllncl3 Ln Ce Nd Sm Gd Binary. Copper Silver Phase Diagram Colored Gold Wikipedia. Copper Silver Phase Diagram Solved 7 Consider The Binary Eutectic Copper Silver Phas. Copper Silver Phase Diagram Ppt Phase Diagrams Powerpoint Presentation Id230040 Abstract. Phase equilibria have been extrapolated to low temperatures, and a condensed phase diagram has been plotted for the Au-Cu system to be consistent with the third law of thermodynamics. Download to read the full article text. Phase diagram of gold-copper: You cannot overwrite this file. File usage. The following 2 pages link to this file: Gold Based Materials; Werkstoffe auf Gold-Basis; Metadata. This file contains additional information, probably added from the digital camera or scanner used to create or digitize it. If the file has been modified from its original ...
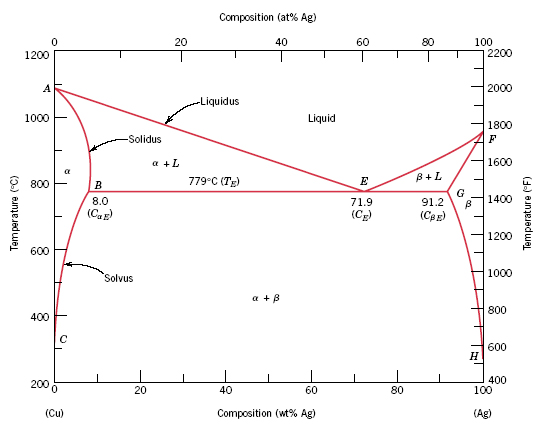
Use the following silver-copper phase diagram for Problems 5-9. 5. What are the solubility limits of Ag in (Cu) and Cu in (Ag)? Recall that (Cu) and (Ag) are the same as α and β, respectively. The solubility limit of Ag in (Cu) is 7.9 wt. % Ag. The solubility limit of Cu in (Ag) is 8.8 wt.% Cu. Note that these Copper wire gage and transformer size for single phase 230 volts electrical motors. Speed of an operating electrical motor with load is lower than the synchronous speed (no load) of the motor. The speed of electrical motors with 2, 4, 6 or 8 poles at 50 Hz and 60 Hz. Aluminum-Copper Phase Diagram Another commonly used phase diagram is the aluminum-copper phase diagram, which is useful for understanding precipitation strengthening in Al-Cu alloys. The amount of copper present in an alloy is plotted on the x-axis. The phase fields of interest are the Al, θ, and Al+θ phase fields on the left hand side. peritectics, and congruent phase transformations for the tin-gold system (Figure 9.36). There are two eutectics on this phase diagram. One exists at 10 wt% Au-90 wt% Sn and 217°C. The reaction upon cooling is L → α + β The other eutectic exists at 80 wt% Au-20 wt% Sn and 280°C. This reaction upon cooling is L → δ + ζ
mp-2258: Cu3Au (cubic, Pm-3m, 221) Edit Crystal. Generate Phase Diagram. Compositional Phase Diagram. Aqueous Stability (Pourbaix) Tags: Gold cupride (1/3) - HT Copper gold (3/1) Gold copper (1/3) CIF VASP POSCAR CSSR JSON.
as axes are called phase diagrams. DEF. A phase diagram (or equilibrium diagram) is a diagram with T and composition as axes, showing the equilibrium constitution. The phase diagram of an alloy made of components A and B, for all combinations of T and X B, defines the A-B system. Binary systems have two components, ternary systems three, and so on.
The copper-gold phase diagram is constructed below. 10.5 Cite the phases that are present and the phase compositions for the following alloys: (a) 25 wt% Pb-75 wt% Mg at 425°C (800°F) (b) 55 wt% Zn-45 wt% Cu at 600°C (1110°F) (c) 7.6 lb m Cu and 144.4 lb m Zn at ...
the thermal equilibrium diagram for the alloy of Copper and Nickel. In order to find what temperature 60% copper solidifies at we simply draw a vertical line from 60% copper until it hits the solidus line and at this is the point where 60% Copper has fully solidified. 0 100 10 90 20 80 30 70 40 60 50 50 60 40 70 30 80 20 90 10 100 0 900 1000 ...
Construct the phase diagram for this system and label each region. (You can use excel or matlab to plot your phase diagram) Question: Given here are the solidus and liquidus temperatures for the copper gold system. Construct the phase diagram for this system and label each region. (You can use excel or matlab to plot your phase diagram)
Feb 24, 2012 · Copper Conductor is the most common material used for electrical wiring. Gold Conductor is used for high-quality surface-to-surface contacts. Silver is the best conductor on the Conductors list. Impure Water is listed in the Conductor List but it has less conductivity. What is the Charge of a Conductor During Carrying Electricity?
Download scientific diagram | Cu-Ag Phase Diagram. The eutectic composition is 28.1 wt% Cu-71.9 wt% Ag and the solid solubility limit of silver in copper is 8 wt% Ag (after Hansen and Anderko 1958 ...
Generate Phase Diagram Compositional Phase Diagram; Aqueous Stability (Pourbaix) Tags: Gold cupride (1/1) Copper gold (1/1) - L1o type Tetraauricupride Gold copper (1/1) Material Details; Final Magnetic Moment 0.001 μ B. Calculated total magnetic moment for the unit cell within the magnetic ordering provided (see below). ...
The Au-Al phase diagram (Figure 14) contains a number of intermetallic compounds formed at compositions situated close to the gold-rich end of the diagram. AuAl2 has a gold content close to that of an 18 carat alloy (75 per cent gold), which makes it possible to produce a hallmarkable 18 carat purple alloy.
phase) Components and Phases Aluminum-Copper Alloy. ... Sugar/Water Phase Diagram S u g a r T e m p e r a t u r e ... concentrations, such as the gold-silver system, have no eutectic. An alloy system that has a eutectic is often referred to as a eutectic system, or eutectic alloy.
Some gold-copper–aluminium alloys form a fine surface texture at heat treatment, yielding an interesting spangling effect. At cooling, they undergo a quasi-martensitic transformation from body-centered cubic to body-centered tetragonal phase; the transformation does not depend on the cooling rate.
Gold-copper nano-alloy, "Tumbaga", in the era of nano: phase diagram and segregation Nano Lett. , 14 ( 11 ) ( 2014 ) , pp. 6718 - 6726 , 10.1021/nl503584q CrossRef View Record in Scopus Google Scholar
192 / Phase Diagrams—Understanding the Basics. A hypothetical ternary phase space diagram made up of metals . A, B, and . C. is shown in Fig. 10.2. This diagram contains two binary eutectics on the two visible faces of the diagram, and a third binary eutectic between ele-ments . B. and . C. hidden on the back of the plot. Because it is ...
The problem with phase diagrams is they become complicated with more than a base metal and one alloy. Figure 1shows a typical silver-copper phase diagram and it tells you a number of things. First, at all temperatures above the liquid line, any combination of silver and copper is liquid.
Solution The copper-gold phase diagram is constructed below. 9.10 Cite the phases that are present and the phase compositions for the following alloys: (a) 15 wt% Sn-85 wt% Pb at 100°C (212°F) (b) 25 wt% Pb-75 wt% Mg at 425°C (800°F) (c) 85 wt% Ag-15 wt% Cu at 800°C (1470°F)
Oct 11, 2018 · Guisbiers, G. et al. Gold-copper nano-alloy, "Tumbaga", in the era of nano: phase diagram and segregation. Nano Lett. 14 , 6718–6726 (2014). CAS Article Google Scholar
Eutectic phase diagram for a silver-copper system. 2800 2600 2400 2200 2000 1800 1600 MgO CaO 20 40 60 80 100 0 C) L MgO ss + L MgO ss CaO ss + L CaO ss MgO ss + CaO ss Wt % Eutetic phase diagram for MgO-CaO system. Temperature (Lecture 19 - Binary phase diagrams 4 of 16 11/23/05
Metals like silver and gold have a difference of 0.2%; nickel and copper of 2.7%, and show complete solid solubility. But zinc and copper have 4.2% difference with maximum solubility of 38.4 wt.% Zn. (other factors are less favourable); Cadmium in copper with 16.5% size difference shows a solid solubility of 1.7 wt.%.
Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash

0 Response to "38 copper gold phase diagram"
Post a Comment