38 consider the sugar-water phase diagram
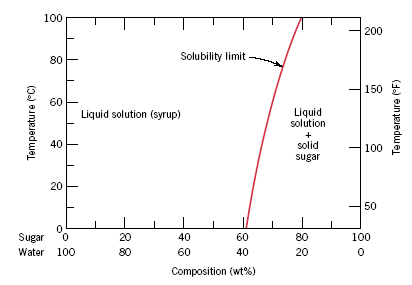
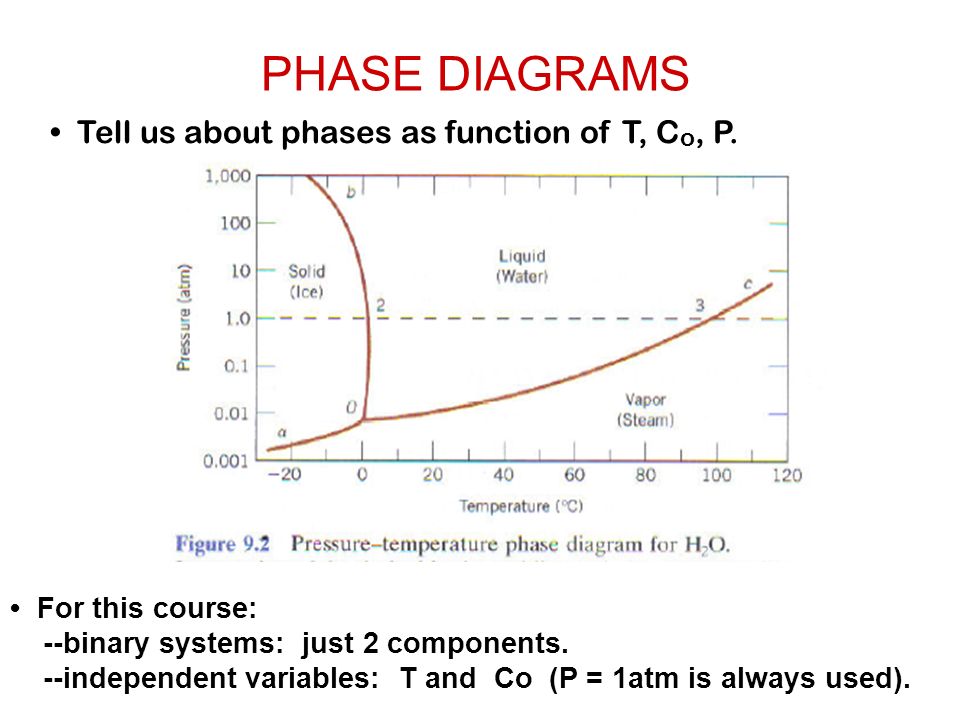
CHAPTER 10 PHASE DIAGRAMS PROBLEM SOLUTIONS. Solubility Limit 10.1 Consider the sugar–water phase diagram of Figure 10.1. (a) How much sugar will dissolve in 1000 g of water at 60°C? (b) If the saturated liquid solution in part (a) is cooled to 20°C, some of the sugar will precipitate out as a solid. Chapter 9 1 Phase Diagrams pendium of Evaluated Constitutional Data and Phase Diagrams, Wiley, New York, 1988—1995. Fifteen volumes. QUESTIONS AND PROBLEMS 9.1 Consider the sugar—water phase diagram of REVISED PAGES EQA Handbook of Ternary Alloy Phase Diagrams, ASM International, Materials Park, OH, 1995. Ten volumes. Also on CD. Composition
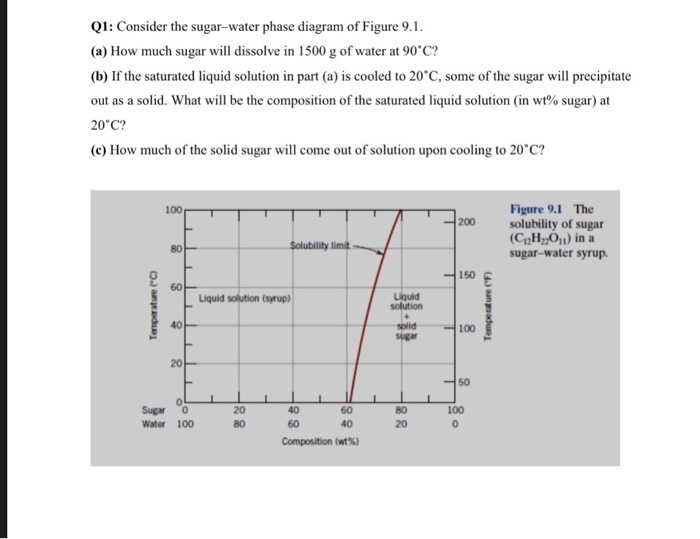
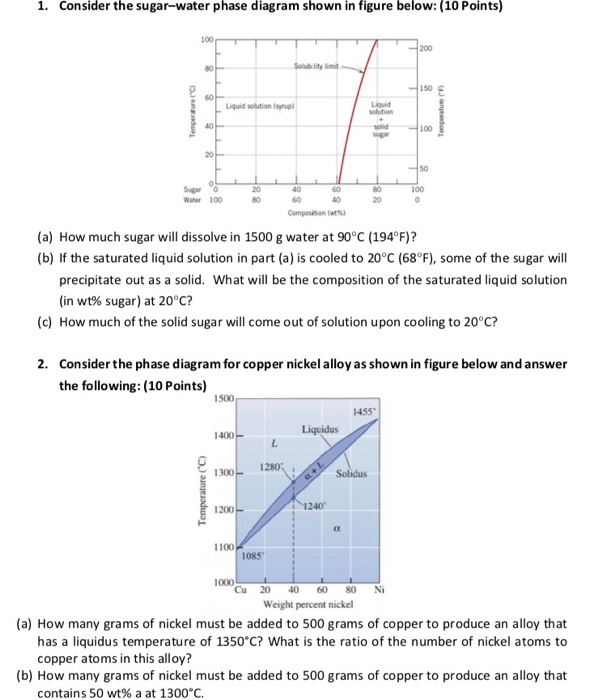
Problem 1. Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1000 g of water at 80 ∘ C ( 176 ∘ F)? (b) If the saturated liquid solution in part (a) is cooled to 20 ∘ C ( 68 ∘ F), some of the sugar will precipitate out as a solid. What will be the composition of the saturated liquid solution (in wt ...

Consider the sugar-water phase diagram
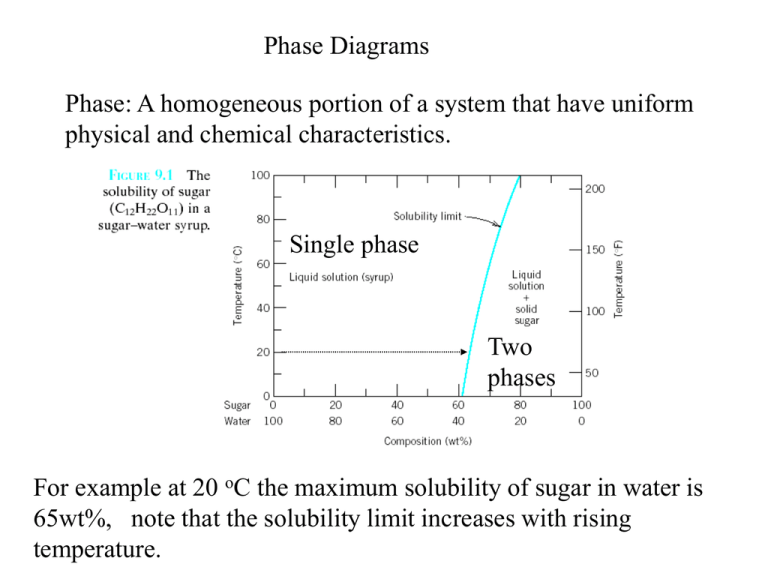
cristobalite, as well as a non-crystalline phase, silica glass, and as molten silica. Every pure material is considered to be a phase, so also is every solid, liquid, and gaseous solution. For example, the sugar-water syrup solution is one phase, and solid sugar is another. 2 MatE 25 (Oye) San Jose State University Fall 2015 9.1 Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1000 g of water at 80°C (176°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid. Review Quiz Question Consider the given sugar water phase diagram How much. Review quiz question consider the given sugar water. School Alfaisal University; Course Title ME 201; Uploaded By student_12340. Pages 15 This preview shows page 13 - 15 out of 15 pages.
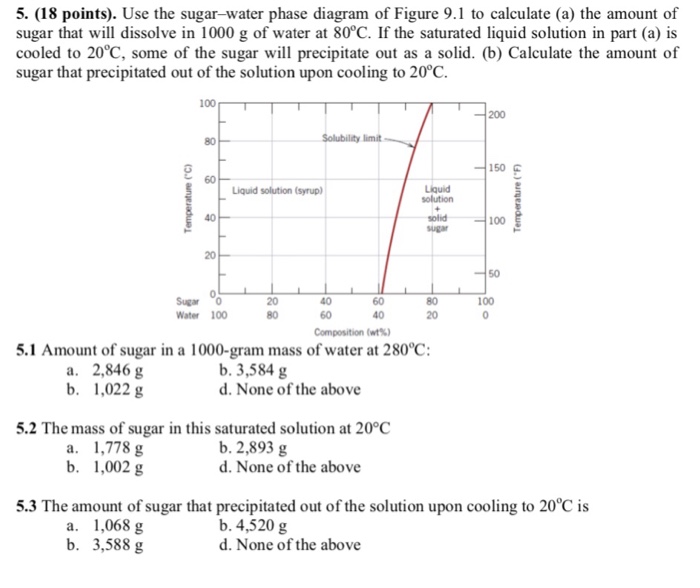
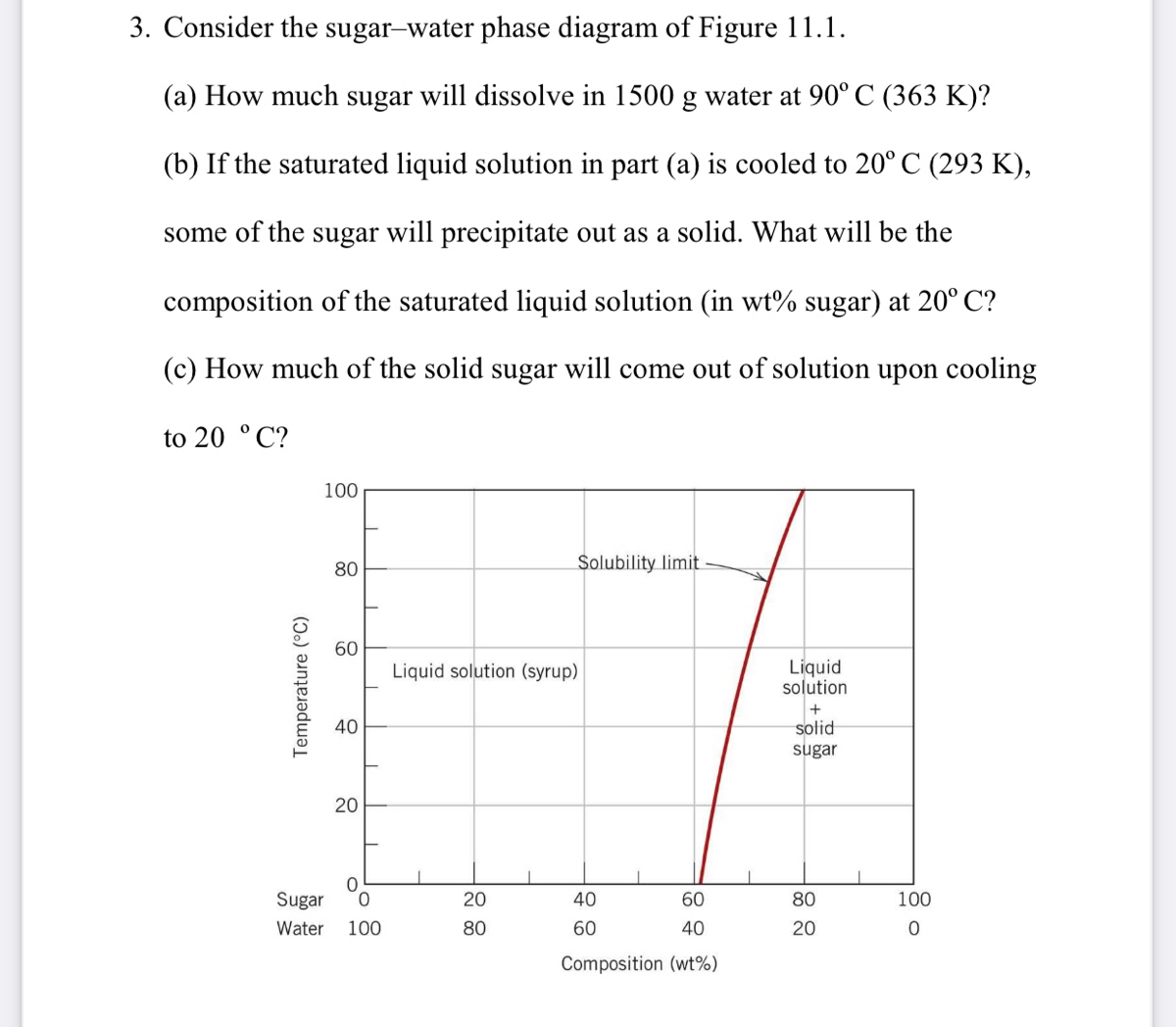
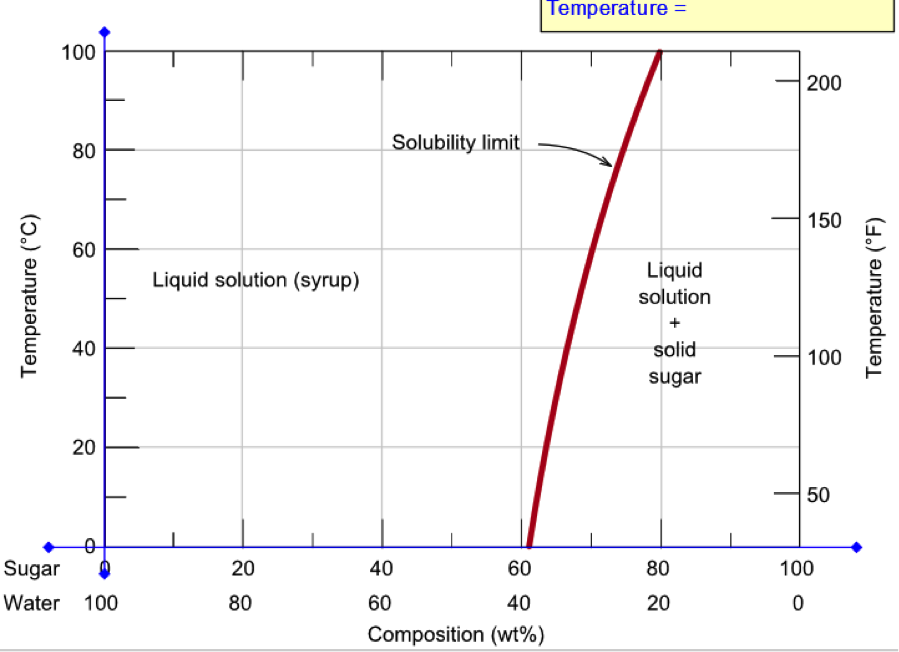
Consider the sugar-water phase diagram. From the solubility limit curve in Figure 9.1, at 90°C the maximum concentration of sugar in the syrup is about 77 wt%. It is now possible to calculate the mass of sugar using Equation 4.3 as × 100 msugar msugar + mwater Csugar (wt%) = × 100 msugar msugar + 1500 g 77 wt% = Solving for msugar yields msugar = 5022 g (b) Again using this same plot, at 20°C the so lubility limit (or the concentration of the saturated solution) is about 64 wt% sugar. 9.1 Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90°C (194°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid. What will be the composition of the saturated liquid solution (in wt% sugar) at 20°C? Question 4 & 5 (see picture for diagram): Consider the following phase diagram of water. The temperature and pressure scales are greatly reduced (and are non-linear). Point A is associated with which of the following? ... A student is preparing a sugar water solution to make rock candy. When the student continues to add sugar to the solution ... PROBLEM SOLUTIONS Solubility Limit 9.1 Consider the sugar- water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 15 00 g water at 90° C (194°F)? (b) If the saturated liquid solution in part (a) is cooled to 20° C (68°F), some of the sugar will precipitate out as a solid. What will be the composition of the saturated liquid solution (in wt% sugar) at 20°C?
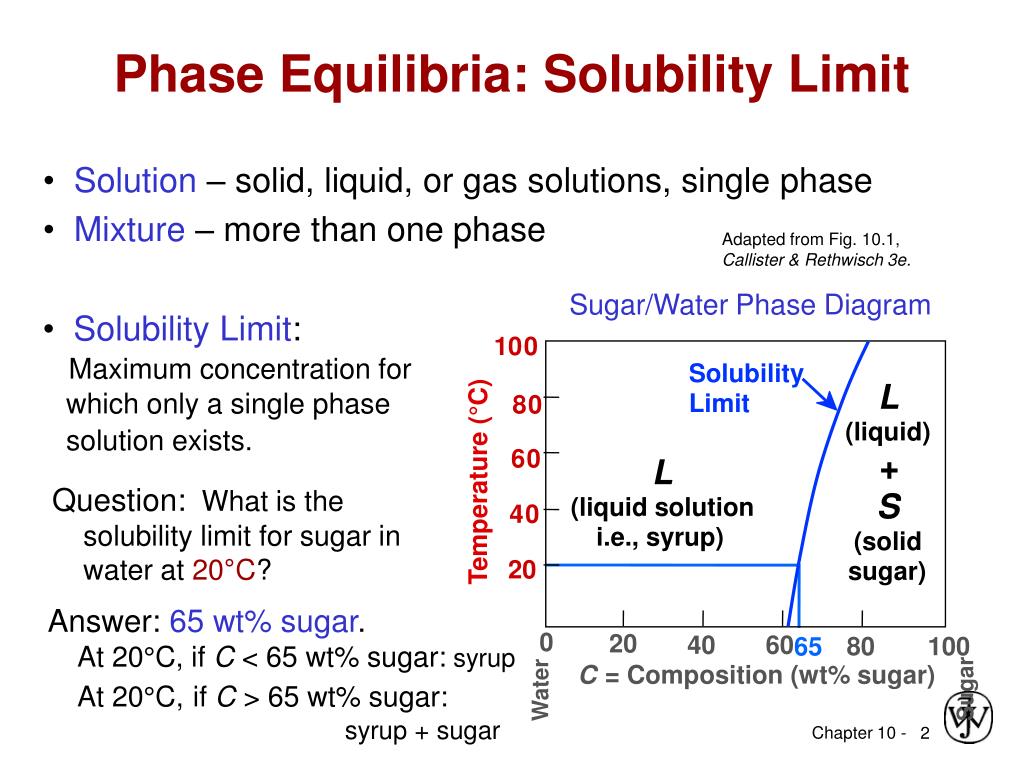
Determining the State of Water Using the phase diagram for water given in , determine the state of water at the following temperatures and pressures: (a) −10 °C and 50 kPa (b) 25 °C and 90 kPa (c) 50 °C and 40 kPa (d) 80 °C and 5 kPa (e) −10 °C and 0.3 kPa (f) 50 °C and 0.3 kPa. Solution Transcribed image text: Consider the sugar-water phase diagram, Animated Figure 10.1: (a) How much sugar will dissolve in 1000 g of water at 70°C? i 5050 g (b) If the saturated liquid solution in part (a) is cooled to 20°C, some of the sugar will precipitate out as a solid, because as seen in the figure, the composition of the saturated liquid solution drops to 64 wt% sugar. Sugar/Water Phase Diagram Sugar Temperature (o C) 0 20 40 60 80 100 C = Composition (wt% sugar) L (liquid solution i.e., syrup) Solubility Limit L (liquid) + S (solid 20 sugar) 40 60 80 100 Water Adapted from Fig. 11.1, Callister & Rethwisch 9e. ... Consider C0= 35 wt% Ni D T D which only a single phase solution exists. Sugar/Water Phase Diagram Sugar Temperature (° C) 0 20 40 60 80 100 C = Composition (wt% sugar) L (liquid solution i.e., syrup) Solubility Limit L (liquid) + S (solid 20 sugar) 40 60 80 100 Water Adapted from Fig. 10.1, Callister & Rethwisch 3e. Chapter 10 - 4 Components and Phases • Components:
Consider the sugar-water phase diagram of Figure. (a) How much sugar will dissolve in 1500 g water at 90°C (194°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid. Phase Equilibria: Solubility Limit 65 Sucrose/Water Phase Diagram Pure Sugar emperature (°C) 0 20 40 60 80 100 C o =Composition (wt% sugar) L (liquid solution i.e., syrup) Solubility Limit L (liquid) + S (solid 20 sugar) 4 0 6 0 8 0 10 0 Pure ater Question: What is the solubility limit at 20C? Answer: 65wt% sugar. If Co < 65wt% sugar: syrup If Co > 65wt% sugar: syrup + sugar. Consider the sugar-water phase diagram, Animated Figure 10.1: (a) How much sugar will dissolve in 1000 g of water at 70°C? (b) If the saturated liquid solution in part (a) is cooled to 20°C, some of the sugar will precipitate out as a solid, because as seen in the figure, the composition of the saturated liquid solution drops to 64 wt% sugar. Consider the sugar - water phase diagram How much sugar will dissolve in 1000 g water at 80°C? If the saturated liquid solution in part (a) is cooled to 20°C, some of the sugar will precipitate out as a solid.
Answer: 65 wt% sugar . If Co < 65 wt% sugar: syrup If Co > 65 wt% sugar: syrup + sugar. 65 Sucrose/Water Phase Diagram Pure Sugar Temperature (°C) 0 20 40 60 80 100 Co =Composition (wt% sugar) L (liquid solution i.e., syrup) Solubility Limit L (liquid) + S (solid 20 sugar) 40 60 80 100 Pure Water Adapted from Fig. 9.1, Callister 7e.

Effect Of Temperature On Ternary Phase Diagrams Of Pyrolytic Lignin Mixed Solvent And Water Sciencedirect
PHASE DIAGRAMS PROBLEM SOLUTIONS Solubility Limit 10.1 Consider the sugar-water phase diagram of Figure 10.1. (a) How much sugar will dissolve in 1000 g of water at 80°C (176°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid.
Q.1. Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90°C (363 K)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (293K), some of the sugar will precipitate out as a solid. What will be the composition of the saturated liquid solution (in wt% sugar) at 20°C? Q.2.

Consider The Sugar Water Phase Diagram Of Figure 91ahow Much Sugar Will Dissolve In 1500 G Water At 90 C 194 F B If The Saturated Liquid Course Hero
9.1 Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90 C (194 F)? (b) If the saturated liquid solution in part (a) is cooled to 20 C (68 F), some of the sugar will precipitate out as a solid. What will be the composition of the saturated liquid solution (in wt% sugar) at 20 C?
Download scientific diagram | The sugar/water phase diagram for the sugar/water system with boiling point line included. Prepared from data referenced in the text. from publication: Building a Low ...
Consider the sugar–water phase diagram below, (a) How much sugar will dissolve in 1000 g of water at 80°C (176°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid.
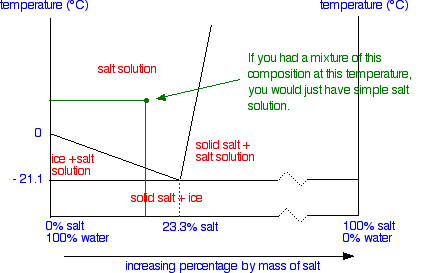
At all temperatures and compositions to the left of this line, there is one phase - a solution of sugar in water. To the right of this line, there are two phases present, the solution and solid sugar (that settles at the bottom of the cup). This could be considered to be a part of the sugar-water phase diagram.
Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1000 g of water at 80°C (176°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid.
Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ...
PHASE DIAGRAMS PROBLEM SOLUTIONS Solubility Limit 9.1 Consider the sugar–water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90 °C (194 °F)? (b) If the saturated liquid solution in part (a) is cooled to 20 °C (68 °F), some of the sugar will precipitate out as a solid.
Consider a sugar-water phase diagram: (a) How much sugar will dissolve in 1000 g of water at 90{eq}^o {/eq} C? (b) If the saturated liquid solution in part (a) is cooled to 20{eq}^o {/eq} C, some ...

Solved Consider The Sugar Water Phase Diagram Of Figure 9 1 A How Much Sugar Will Dissolve In 1500 G Water At 90 9 194 F If The Saturated Liquid Solution In Part A
CHAPTER 9 PHASE DIAGRAMS PROBLEM SOLUTIONS Solubility Limit 9.1 Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g water at 90°C (363 K)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (293 K), some of the sugar will precipitate out as a solid.
8.1 Phase Diagram of Water • Field - 1 phase • Line - phase coexistence, 2 phases ... Consider the binary equilibrium phase diagram of elements A and B that are completely soluble in each other C o ... sugar) • Changing T can change # of phases: path A to B.
Mechanical Engineering. Mechanical Engineering questions and answers. 9.1 Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1500 g of water at 90°C (194°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid.
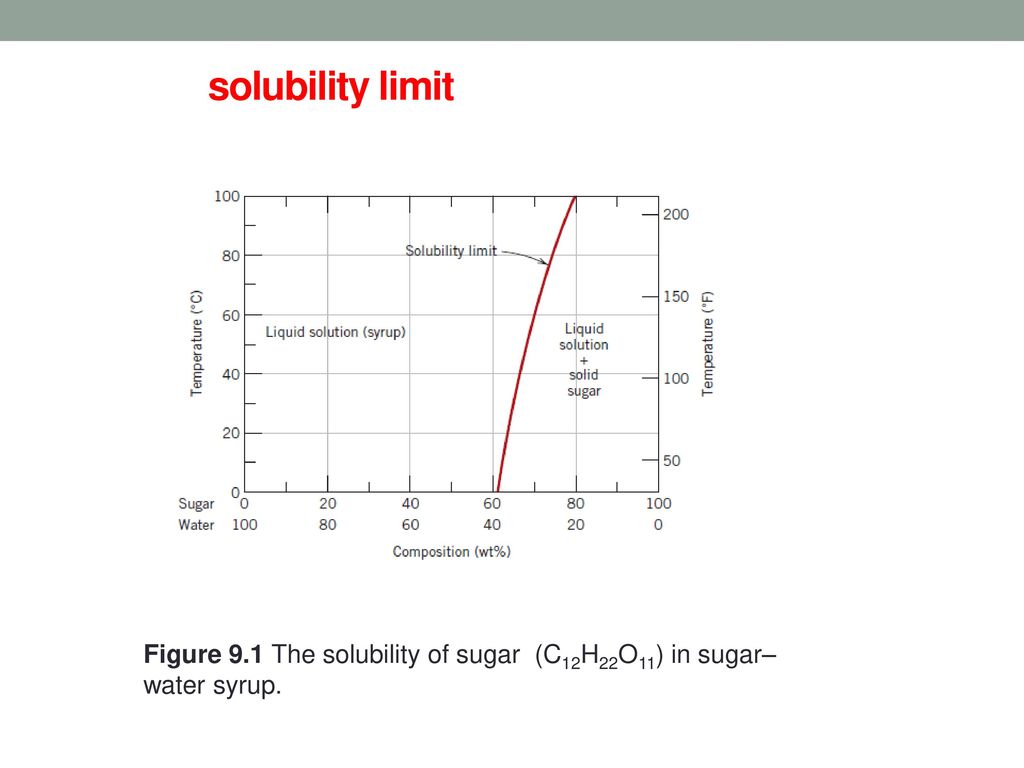
Consider the sugar-water phase diagram of Figure 10.1. Figure 10.1 The solubility of sugar (C 12 H 22 O 11) in a sugar-water syrup. (a) How much sugar will dissolve in 1000 g of water at 80?C (176?F)? (b) If the saturated liquid solution in part (a) is cooled to 20?C (68?F), some of the sugar will precipitate out as a solid.
Review Quiz Question Consider the given sugar water phase diagram How much. Review quiz question consider the given sugar water. School Alfaisal University; Course Title ME 201; Uploaded By student_12340. Pages 15 This preview shows page 13 - 15 out of 15 pages.
MatE 25 (Oye) San Jose State University Fall 2015 9.1 Consider the sugar-water phase diagram of Figure 9.1. (a) How much sugar will dissolve in 1000 g of water at 80°C (176°F)? (b) If the saturated liquid solution in part (a) is cooled to 20°C (68°F), some of the sugar will precipitate out as a solid.
cristobalite, as well as a non-crystalline phase, silica glass, and as molten silica. Every pure material is considered to be a phase, so also is every solid, liquid, and gaseous solution. For example, the sugar-water syrup solution is one phase, and solid sugar is another. 2
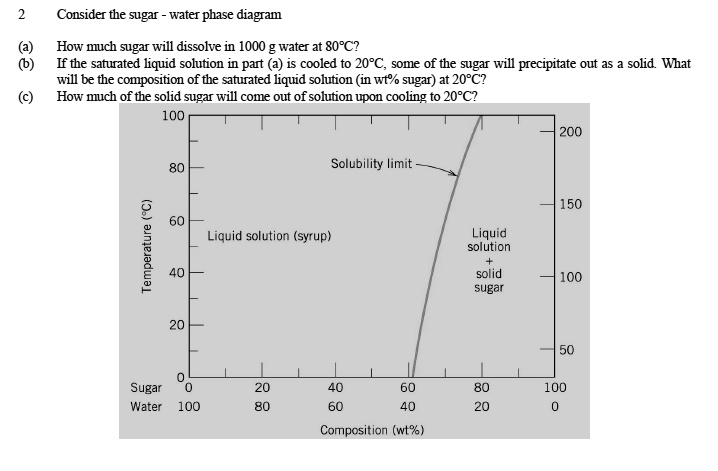
Solved Consider The Sugar Water Phase Diagram How Nuch Sugar Wll Dissolve In 1000 Water 80pc If The Saturated Liquid Solution In Part Is Cooled To 20pc Some Of The Sugar Will

Consider A Sugar Water Phase Diagram A How Much Sugar Will Dissolve In 1000 G Of Water At 90 O C B If The Saturated Liquid Solution In Part A Is Cooled To

Effect Of Temperature On Ternary Phase Diagrams Of Pyrolytic Lignin Mixed Solvent And Water Sciencedirect











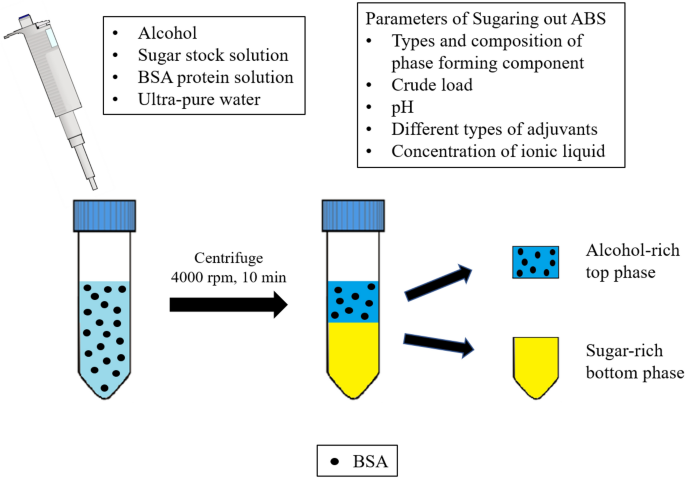
0 Response to "38 consider the sugar-water phase diagram"
Post a Comment