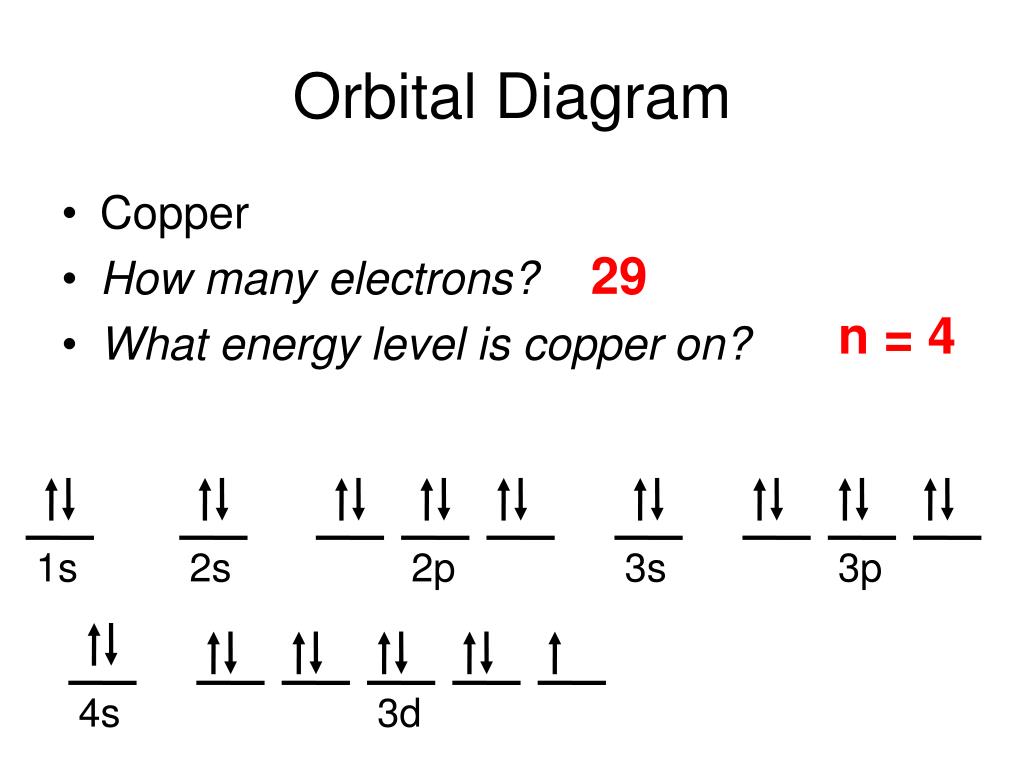
37 orbital diagram for copper
Orbital diagram of Copper (Cu) 30: Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium (Se) 35: Orbital diagram of Bromine (Br) 36: Orbital diagram of Krypton (Kr) 37: Orbital diagram of Rubidium (Rb) 38: Orbital diagram of ... Copper is in the ninth column of the transition metals in the d block of the fourth energy level of the periodic table. This would make the electron configuration for copper, 1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^9 or in noble gas configuration [Ar] 4s^2 3d^9. However, because the 3d orbital is so much larger then the 4s orbital and the 3d orbital only needs one more electron to be filled, the 3d ...
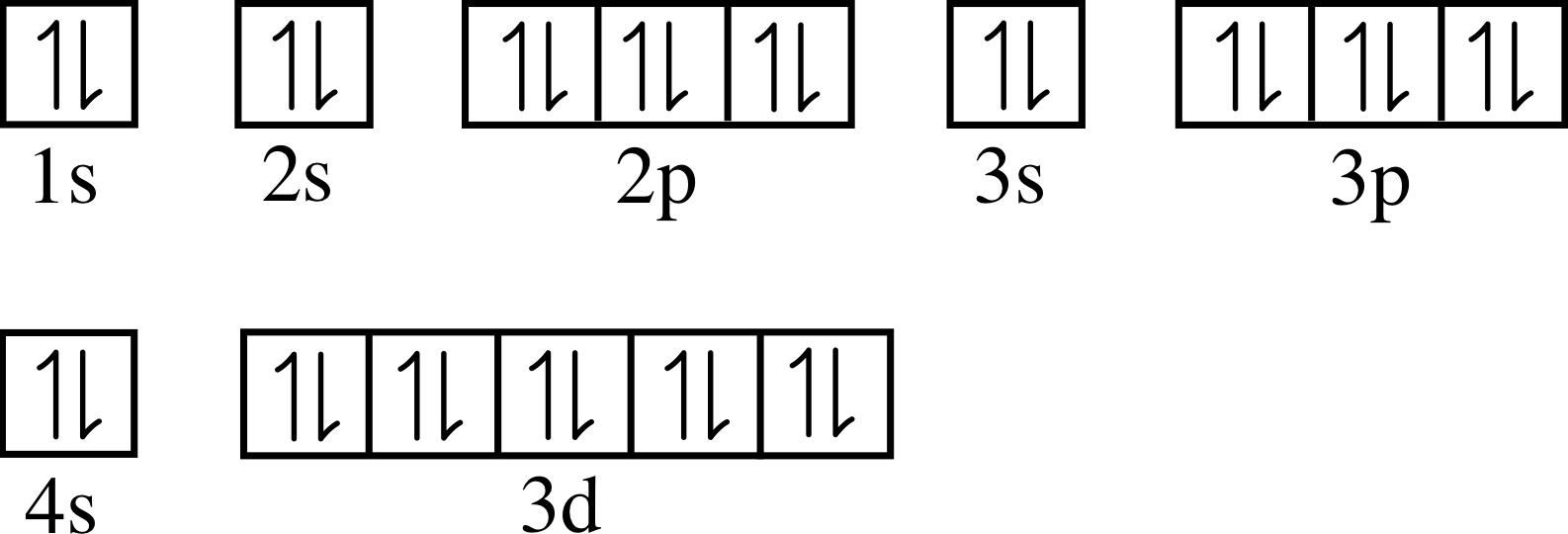
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal –each electron occupies the lowest energy orbital available; German for “build up” •Electrons are notated with an arrow –Up arrow goes first then, down arrow –Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom

Orbital diagram for copper
The orbital diagram for copper is given by, Here in the above diagram, electrons filled in orbitals according to Aufbau principle, which states that orbitals with lower energy are filled first and ... The electron configuration of copper is 1s22s22p63s23p63d104s1. An orbital is a region of space that an electron can exist in. For the diagram you start with the 1 s orbital and then 2s, 2p, and ... In writing the electron configuration for Copper the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Copper go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two ...
Orbital diagram for copper. And you find that for copper, #Z_"the atomic number"=29#. Now #Z# represents the CHARACTERISTIC number of protons, massive, positively-charged nuclear particles that are found in an element's nucleus. For copper #Z=29#.Of course, the nucleus also contains 30-35 neutrons, massive, NEUTRALLY-charged nuclear particles, which gives rise to an isotopic distribution, but these nuclear phenomena do ... Write the electron configuration of copper. The electronic configuration of an element is a symbolic notation of the manner in which the electrons of its atoms are distributed over different atomic orbitals. While writing electron configurations, a standardized notation is followed in which the energy level and the type of orbital are written ... Start by drawing its orbital notation for the outermost, valence electrons. [Ne] ↑↓ ↑↓ ↑ ↑ 3s 3p Sulfur is a nonmetal and tends to gain electrons, creating the -2 charge. Gaining two electrons gives it an octet of 3s23p6. • Copper has two common oxidation states, +1 and +2. The order of the orbital occupancy is shown in the following diagram. According to Aufbau Principle, the first two electrons of Copper (Cu) are filled in 1s orbital. Then, the next two electrons for Copper (Cu) occupy the 2s orbital. And, next 6 electrons for Copper (Cu) will go to in the 2p orbital. Then, the next two electrons go in the 3s.
In writing the electron configuration for Copper the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Copper go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two ... The electron configuration of copper is 1s22s22p63s23p63d104s1. An orbital is a region of space that an electron can exist in. For the diagram you start with the 1 s orbital and then 2s, 2p, and ... The orbital diagram for copper is given by, Here in the above diagram, electrons filled in orbitals according to Aufbau principle, which states that orbitals with lower energy are filled first and ...

Or Exam 2 Material Saved Omplete The Partial Orbital Diagrams For The Elements Indica Fill From Left To Right And Homeworklib

Orbital Notations And Electron Configurations 1 2 N Principle Energy Level 2n 2 Of E In N Ppt Download
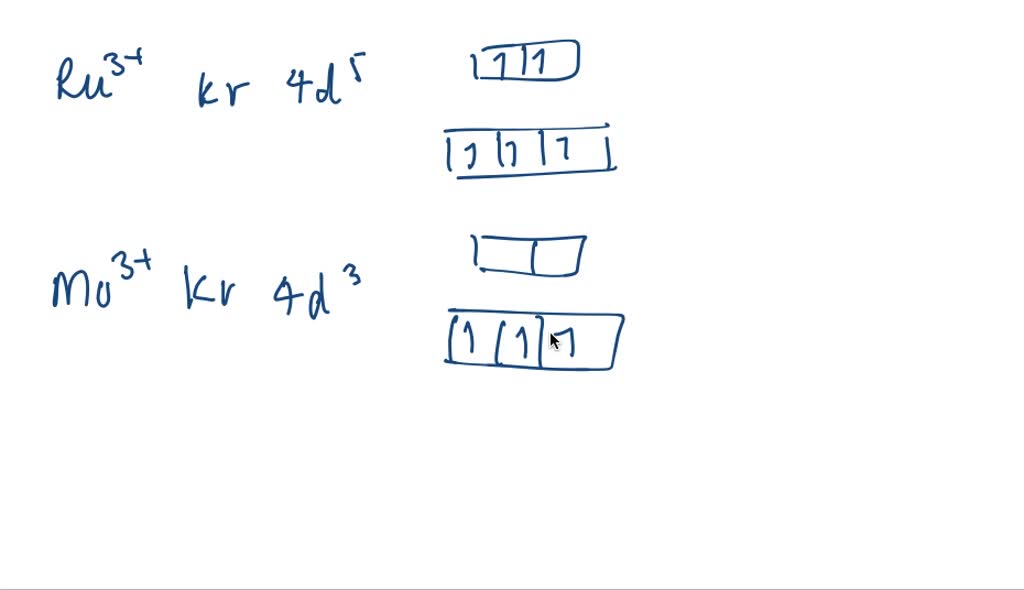
Solved 2 Consider The Neutral Atom For The Element Of Copper A Write The Noble Gas Configuration And Orbital Diagram Based On The Noble Gas Config For A Neutral Atom Of Copper Atom

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes







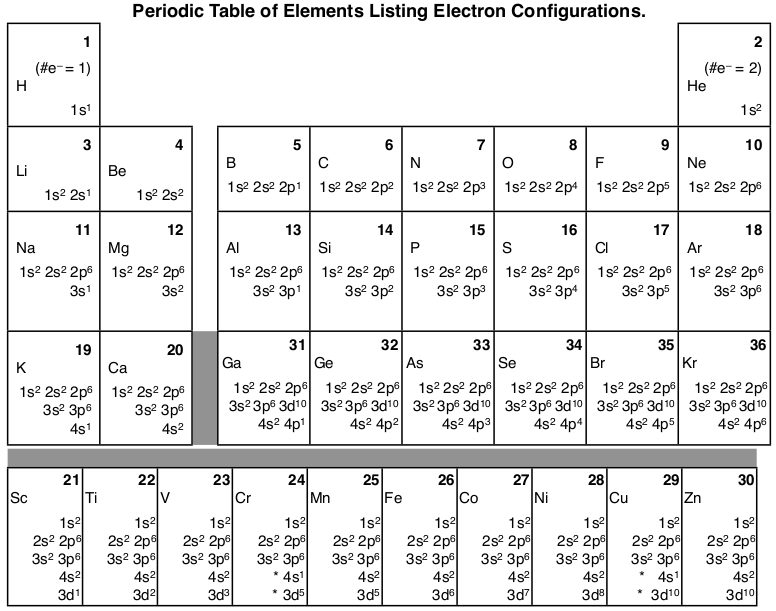



/aufbauexample-56a129555f9b58b7d0bc9f48.jpg)










0 Response to "37 orbital diagram for copper"
Post a Comment