37 how many electrons are depicted in the electron dot diagram
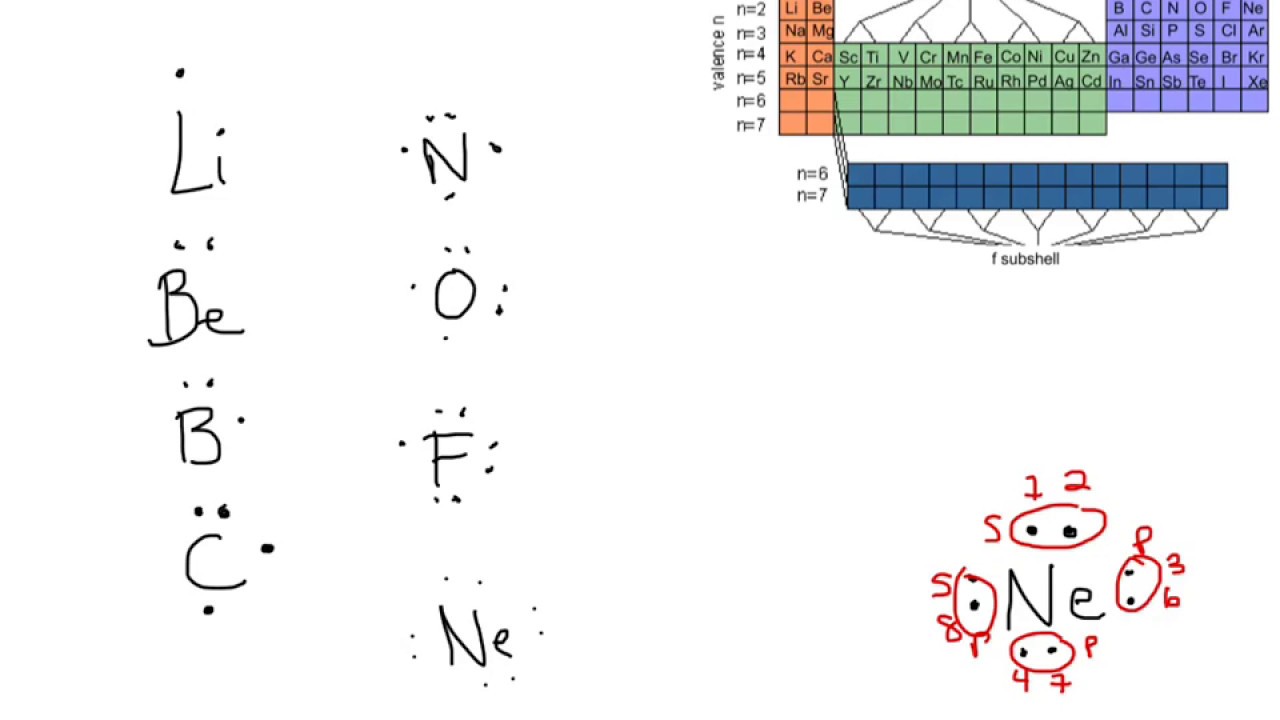
The former, known as a 'Lewis dot diagram,' indicates a pair of shared electrons between the atomic symbols, while the latter, known as a 'Lewis structure,' uses a dash to indicate the pair of shared electrons that form a covalent bond. More complicated molecules are depicted this way as well. The electron dot diagram of elements or ions depict only the valence electrons. Carbon has atomic number of 6. The first shell has 2 electrons and gets filled. The second shell is filled by the remaining four electrons.The 4 electrons of the valence shell is shown in the electron dot diagram.
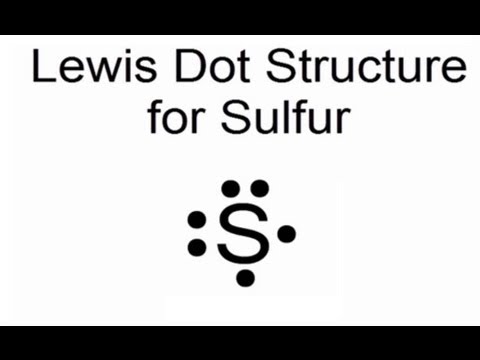
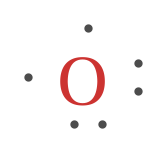
A Lewis structure is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell. As an example, an oxygen atom has six electrons in its outer shell. In a Lewis structure, these six dots are arranged so that an atom has two lone pairs and two single electrons.

How many electrons are depicted in the electron dot diagram
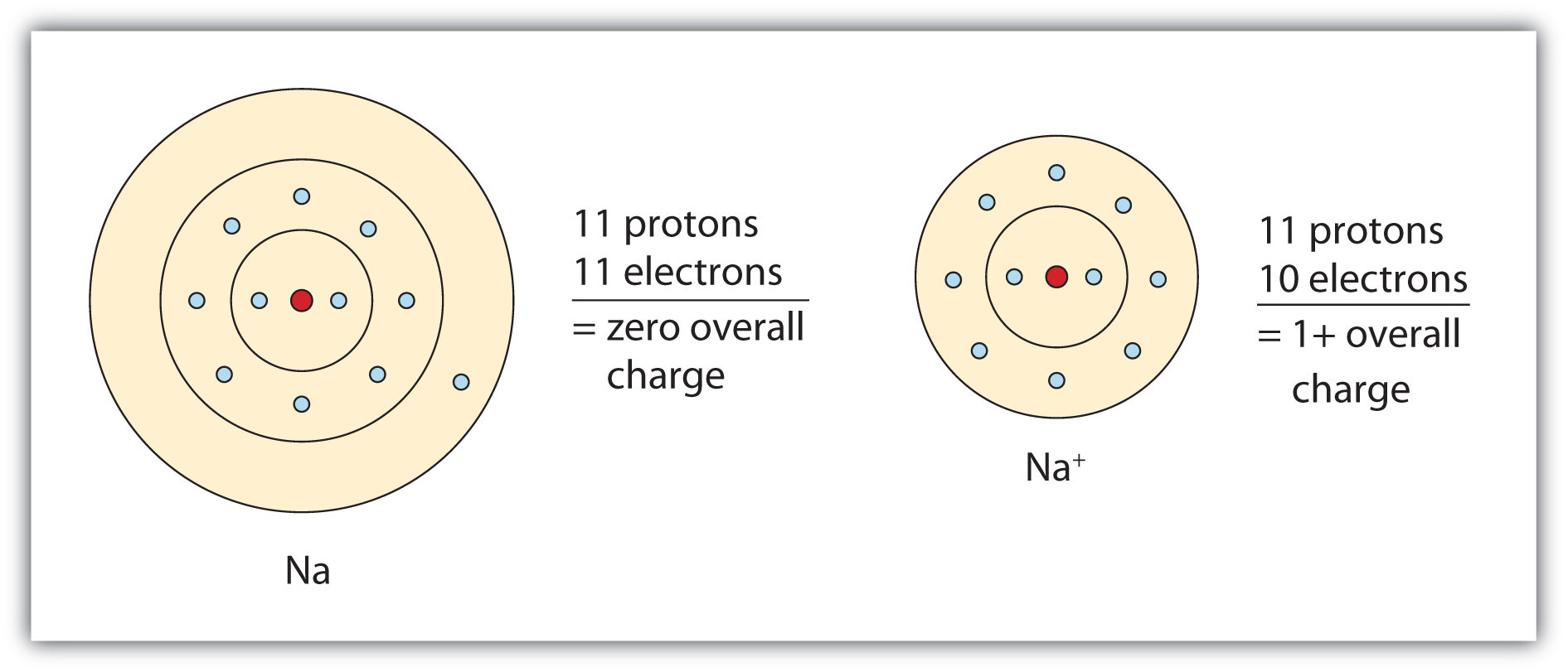
Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1. Answer (1 of 3): Think about the nature of these elements: Carbon is unique in that it usually forms 4 bonds, while chlorine and fluoride are both halogens (usually forming 1 bond). Add the total valence electrons from each: 4 + 2(7) + 2(7) = 32 total ... In the Lewis symbol, the electrons are depicted as two lone pair dots. What is the dot diagram? A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence ...
How many electrons are depicted in the electron dot diagram. How many electrons should be represented in the Lewis dot structure for nitrogen from ECON 1101 at Kennesaw State University 180 seconds. Q. The electron configuration of an atom is 1s 2 2s 2 2p 6. The number of electrons in the atom is. answer choices. 3. 6. 8. 10. To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s 2 2s 2 2p 6). Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level. According to the periodic table, how many protons and electrons does an atom of argon (Ar) contain? answer choices. 18 protons, 40 electrons. 18 protons, 18 electrons. 20 protons, 18 electrons. 40 protons, 20 electrons. <p>18 protons, 40 electrons</p>. alternatives.
The Lewis Dot Structure is a visual which represents the outermost shell of electrons, also known as valence electrons, and possible covalent bonds within an atom or molecule. These valence electrons are negatively charged and are attracted to the positively charged nucleus, made up of neutrons and protons. Answer: I will explain this with pictures, and some captions. This is just the five atoms in CH4, or Methane. I have drawn them above. The red one in the middle is the one Carbon, and the four yellow ones are Hydrogens. Now I have drawn the Valence Electrons. These are the blue dots next to the... How many electrons are depicted in the electron dot diagram of an electrically neutral carbon atom? 4 What feature of a chemical equation represents the law of conservation of matter? Considering this, what is the electron dot diagram for Helium? The Lewis symbol for helium: Helium is one of the noble gases and contains a full valence shell. Unlike the other noble gases in Group 8, Helium only contains two valence electrons.In the Lewis symbol, the electrons are depicted as two lone pair dots.. Beside above, what is the purpose of an electron dot diagram?
How many electrons are depicted in the electron dot diagram of an electrically neutral oxygen atom? six. When you balance a chemical equation, what are you doing? ... 20 protons, 20 electrons, 24 neutrons. THIS SET IS OFTEN IN FOLDERS WITH... k12 Chemistry Semester 1 Final Practice Test. 50 terms. How to Draw a Lewis Structure. Step 1: Find the Total Number of Valence Electrons. …. Step 2: Find the Number of Electrons Needed to Make the Atoms "Happy" …. Step 3: Determine the Number of Bonds in the Molecule. …. Step 4: Choose a Central Atom. …. Step 5: Draw a Skeletal Structure. …. Step 6: Place Electrons Around Outside Atoms. An electron 13, Electron dot diagram for boron. The unpaired electron is usually placed in the Lewis Dot Structure so The problem with this structure is that boron has an incomplete octet;. Structure, properties, spectra, suppliers and links for: Boron nitride. Boron has 5 electrons. 3 are in the valence shell. BHow many electrons should be shown in the Lewis dot structure for hydrogen. Because hydrogen only needs two electrons to fill its valence shell it is an exception to the octet rule. Report an issue. 1 Nitrogen atom needs 3 electrons and all 3 Hydrogen atoms need 1 more electron to get stable.

Solved How Many Electrons Are Shown In Each Element S Electron Dot Structure A Carbon C Calcium B Iodine D Gallium
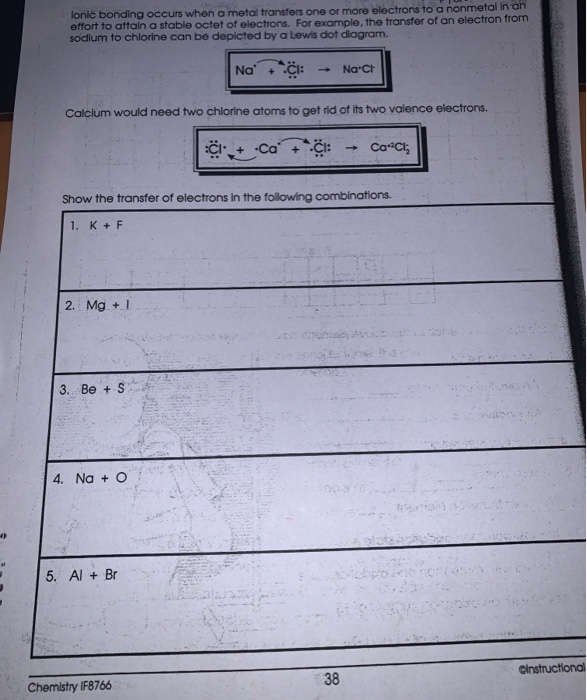
How to Draw a Lewis Dot Structure Step 1. Determine the total number of valence electrons to be depicted in the Lewis diagram. Example: CO 2 Total = 16. Step 2. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Step 3. Determine how many electrons must be added to central element.
How do you write the electron dot structure of an element? Determine the total number of valence electrons to be depicted in the Lewis diagram. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Determine how many electrons must be added to central element.
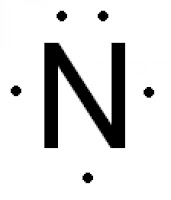
number of protons = number of electrons. Hence, N atom has 7 electrons. - The electron configuration is 1s² 2s² 2p³. Hence, N atom has 2 + 3 = 5 valence electrons. So, five electrons are represented in electron dot diagram of N.
To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s22s22p6). Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level.

Draw The Lewis Dot Structure For The Covalent Molecule N2o4 Adding Formal Charges Where Necessary Study Com
How to Draw a Lewis Dot Structure. Determine the total number of valence electrons to be depicted in the Lewis diagram. Place least electronegative element in center and draw single bonds from the central atom to other atoms. Determine how many electrons must be added to central element.
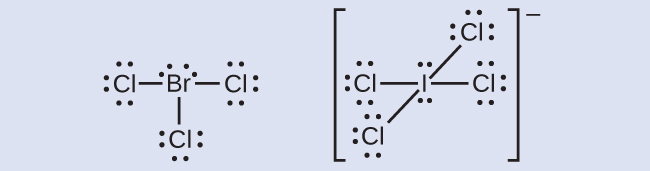
Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
In the Lewis symbol, the electrons are depicted as two lone pair dots. What is the dot diagram? A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence ...
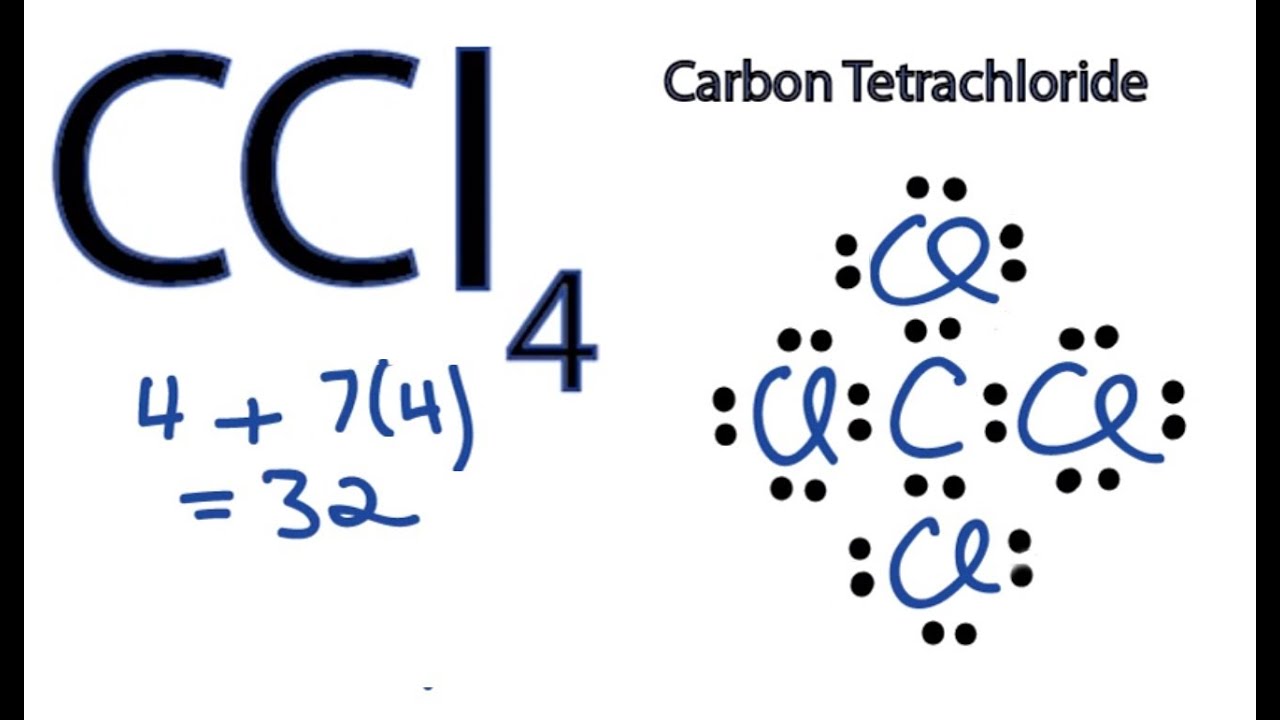
Answer (1 of 3): Think about the nature of these elements: Carbon is unique in that it usually forms 4 bonds, while chlorine and fluoride are both halogens (usually forming 1 bond). Add the total valence electrons from each: 4 + 2(7) + 2(7) = 32 total ...
Solution. Having lost its two original valence electrons, the Lewis electron dot diagram is just Ca 2+. Ca2+. The O 2− ion has gained two electrons in its valence shell, so its Lewis electron dot diagram is as follows: Test Yourself. The valence electron configuration of thallium, whose symbol is Tl, is 6 s2 5 d10 6 p1.

Lewis Dot Structures Dots Are Arranged Around An Element S Symbol To Indicate The Valence Electrons Reminder Valence Shell Only Has S P Orbitals Maximum Ppt Download








:max_bytes(150000):strip_icc()/SulfurOctetRule-56a12a2c3df78cf77268035f.png)


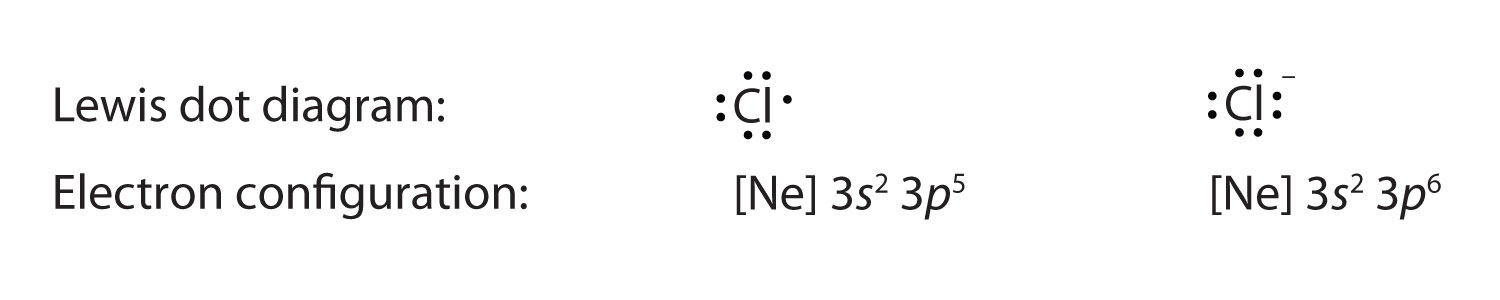








0 Response to "37 how many electrons are depicted in the electron dot diagram"
Post a Comment